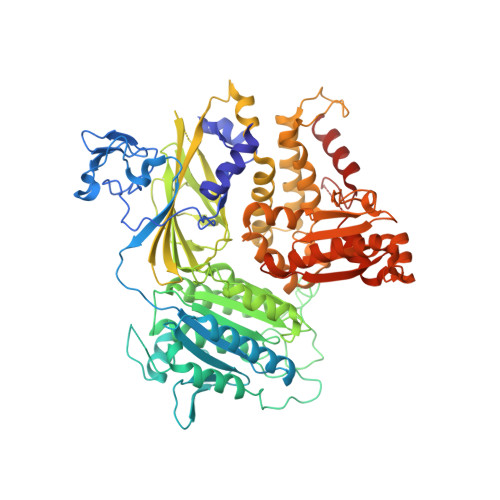
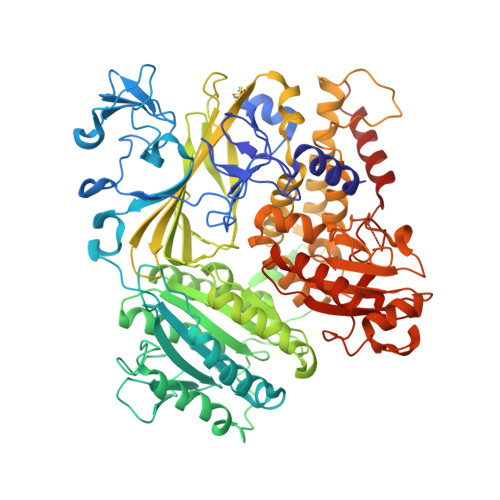
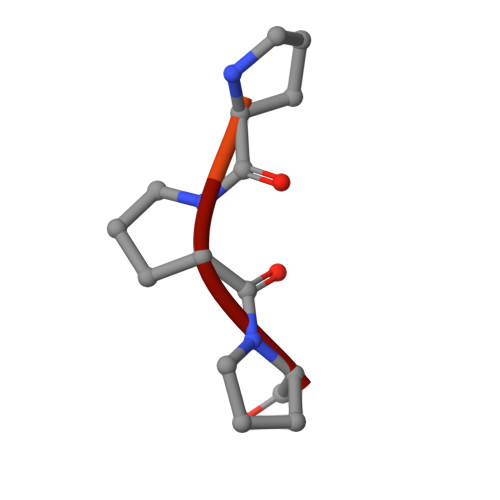
TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats.
Ma, W., Goldberg, J.(2016) Proc Natl Acad Sci U S A 113: 10061-10066
- PubMed: 27551091
- DOI: https://doi.org/10.1073/pnas.1605916113
- Primary Citation of Related Structures:
5KYN, 5KYU, 5KYW, 5KYX, 5KYY - PubMed Abstract:
The supramolecular cargo procollagen is loaded into coat protein complex II (COPII)-coated carriers at endoplasmic reticulum (ER) exit sites by the receptor molecule TANGO1/cTAGE5. Electron microscopy studies have identified a tubular carrier of suitable dimensions that is molded by a distinctive helical array of the COPII inner coat protein Sec23/24•Sar1; the helical arrangement is absent from canonical COPII-coated small vesicles. In this study, we combined X-ray crystallographic and biochemical analysis to characterize the association of TANGO1/cTAGE5 with COPII proteins. The affinity for Sec23 is concentrated in the proline-rich domains (PRDs) of TANGO1 and cTAGE5, but Sec23 recognizes merely a PPP motif. The PRDs contain repeated PPP motifs separated by proline-rich linkers, so a single TANGO1/cTAGE5 receptor can bind multiple copies of coat protein in a close-packed array. We propose that TANGO1/cTAGE5 promotes the accretion of inner coat proteins to the helical lattice. Furthermore, we show that PPP motifs in the outer coat protein Sec31 also bind to Sec23, suggesting that stepwise COPII coat assembly will ultimately displace TANGO1/cTAGE5 and compartmentalize its operation to the base of the growing COPII tubule.
- Howard Hughes Medical Institute, Memorial Sloan Kettering Cancer Center, New York, NY 10065; Structural Biology Program, Memorial Sloan Kettering Cancer Center, New York, NY 10065.
Organizational Affiliation: