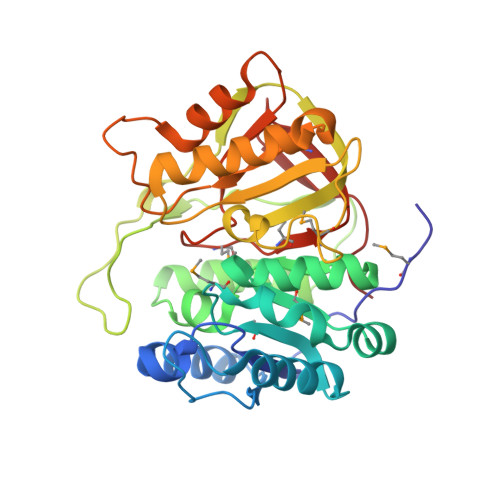
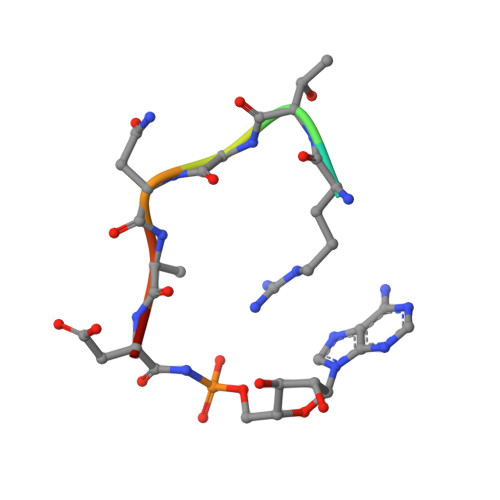
Crystal structure of microcin immunity protein MccF from Bacillus anthracis in complex with McC
Nocek, B., Kulikovsky, A., Severinov, K., Dubiley, S., Joachimiak, A., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.