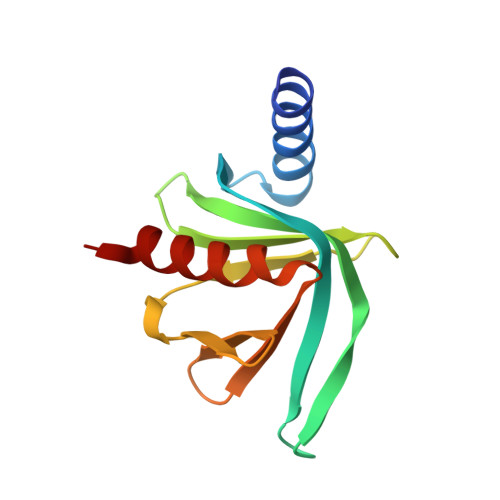
The S. pombe mRNA decapping complex recruits cofactors and an Edc1-like activator through a single dynamic surface.
Wurm, J.P., Overbeck, J., Sprangers, R.(2016) RNA 22: 1360-1372
- PubMed: 27354705
- DOI: https://doi.org/10.1261/rna.057315.116
- Primary Citation of Related Structures:
5JP4 - PubMed Abstract:
The removal of the 5' 7-methylguanosine mRNA cap structure (decapping) is a central step in the 5'-3' mRNA degradation pathway and is performed by the Dcp1:Dcp2 decapping complex. The activity of this complex is tightly regulated to prevent premature degradation of the transcript. Here, we establish that the aromatic groove of the EVH1 domain of Schizosaccharomyces pombe Dcp1 can interact with proline-rich sequences in the exonuclease Xrn1, the scaffolding protein Pat1, the helicase Dhh1, and the C-terminal disordered region of Dcp2. We show that this region of Dcp1 can also recruit a previously unidentified enhancer of decapping protein (Edc1) and solved the crystal structure of the complex. NMR relaxation dispersion experiments reveal that the Dcp1 binding site can adopt multiple conformations, thus providing the plasticity that is required to accommodate different ligands. We show that the activator Edc1 makes additional contacts with the regulatory domain of Dcp2 and that an activation motif in Edc1 increases the RNA affinity of Dcp1:Dcp2. Our data support a model where Edc1 stabilizes the RNA in the active site, which results in enhanced decapping rates. In summary, we show that multiple decapping factors, including the Dcp2 C-terminal region, compete with Edc1 for Dcp1 binding. Our data thus reveal a network of interactions that can fine-tune the catalytic activity of the decapping complex.
- Max Planck Institute for Developmental Biology, 72076 Tübingen, Germany.
Organizational Affiliation: