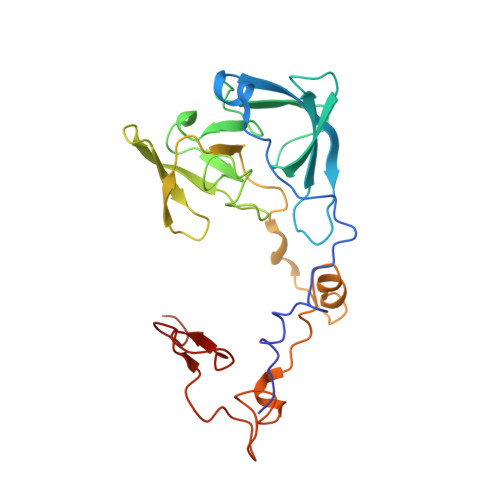
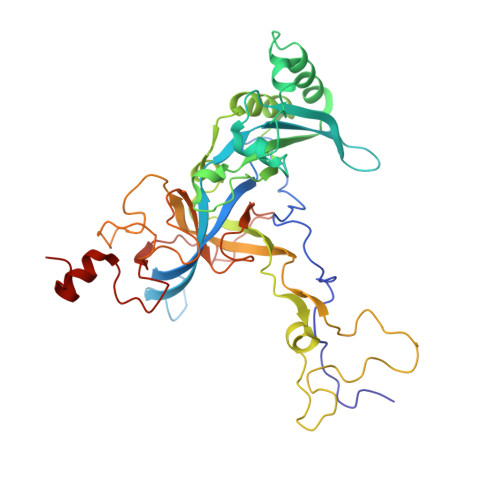
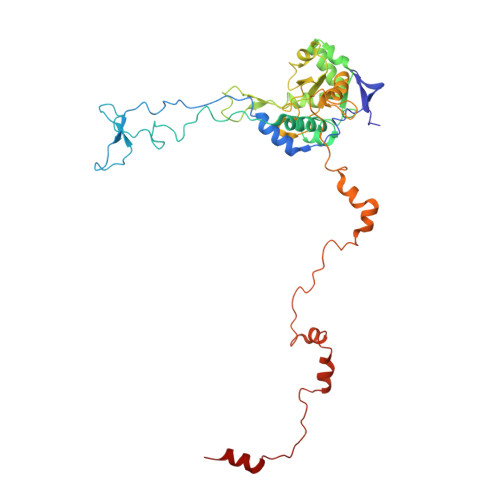
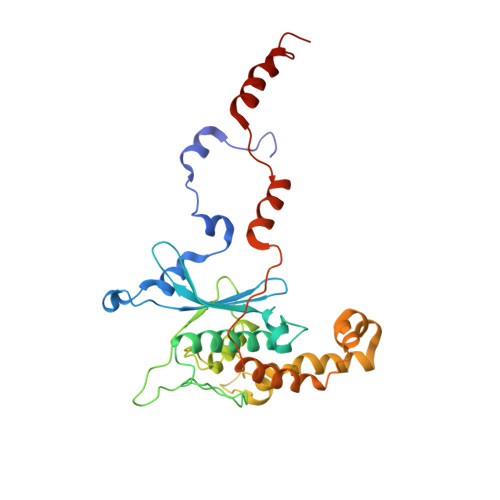
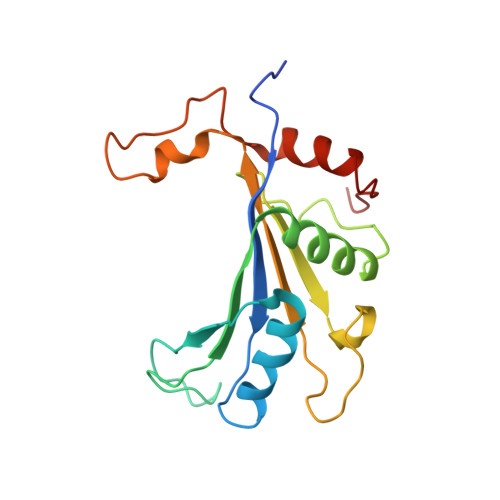
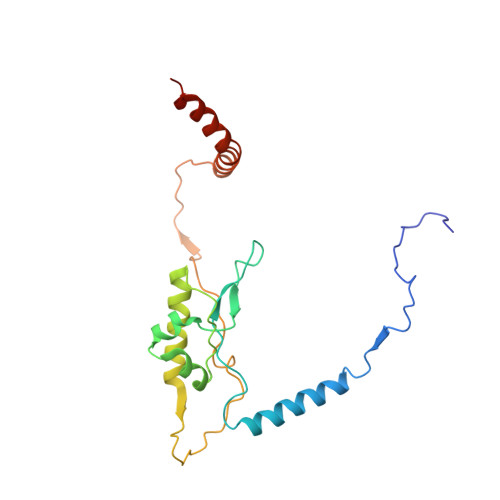
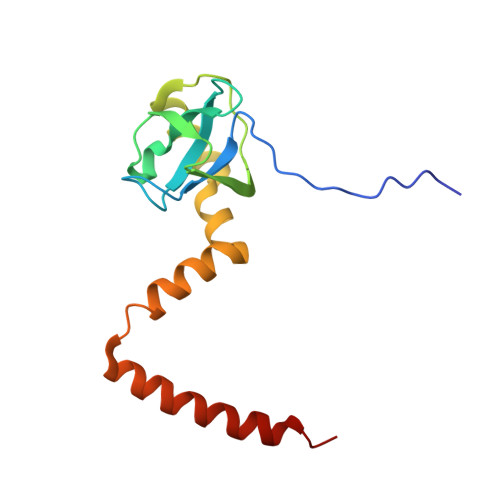
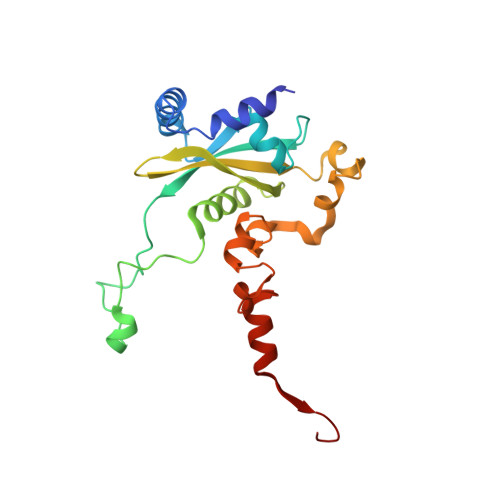
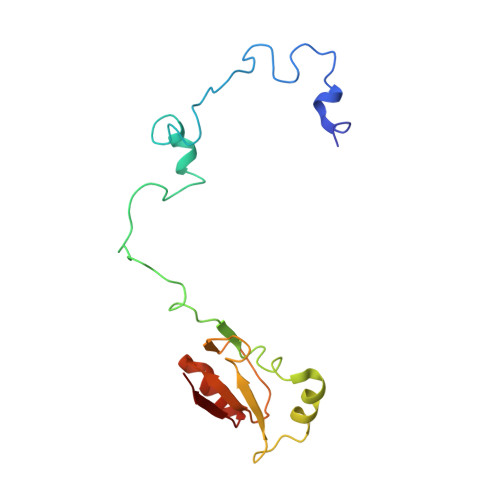
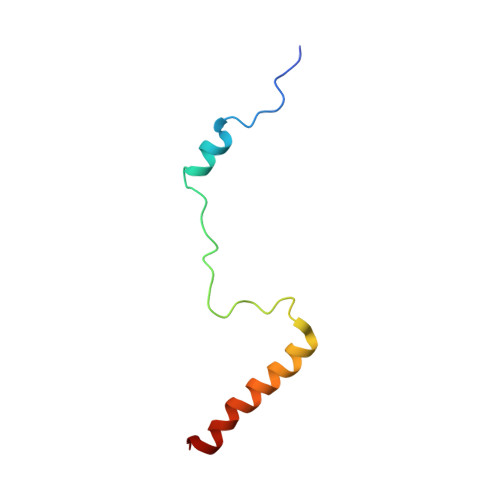
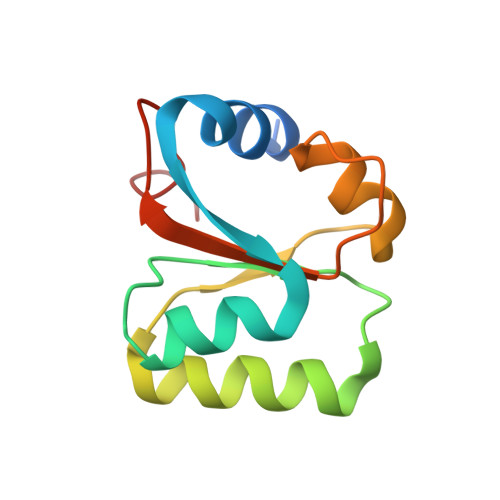
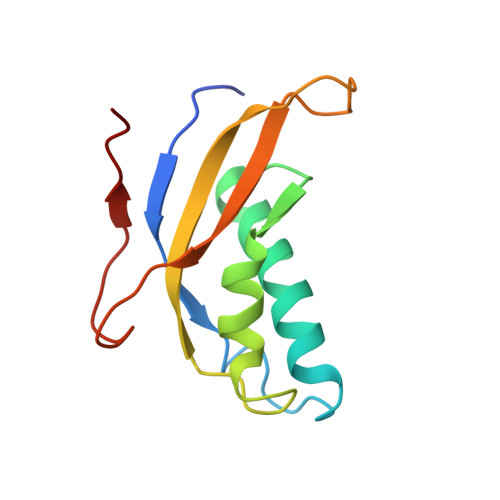
Structural snapshot of cytoplasmic pre-60S ribosomal particles bound by Nmd3, Lsg1, Tif6 and Reh1
Ma, C., Wu, S., Li, N., Chen, Y., Yan, K., Li, Z., Zheng, L., Lei, J., Woolford, J.L., Gao, N.(2017) Nat Struct Mol Biol 24: 214-220
- PubMed: 28112732
- DOI: https://doi.org/10.1038/nsmb.3364
- Primary Citation of Related Structures:
5H4P - PubMed Abstract:
A key step in ribosome biogenesis is the nuclear export of pre-ribosomal particles. Nmd3, a highly conserved protein in eukaryotes, is a specific adaptor required for the export of pre-60S particles. Here we used cryo-electron microscopy (cryo-EM) to characterize Saccharomyces cerevisiae pre-60S particles purified with epitope-tagged Nmd3. Our structural analysis indicates that these particles belong to a specific late stage of cytoplasmic pre-60S maturation in which ribosomal proteins uL16, uL10, uL11, eL40 and eL41 are deficient, but ribosome assembly factors Nmd3, Lsg1, Tif6 and Reh1 are present. Nmd3 and Lsg1 are located near the peptidyl-transferase center (PTC). In particular, Nmd3 recognizes the PTC in its near-mature conformation. In contrast, Reh1 is anchored to the exit of the polypeptide tunnel, with its C terminus inserted into the tunnel. These findings pinpoint a structural checkpoint role for Nmd3 in PTC assembly, and provide information about functional and mechanistic roles of these assembly factors in the maturation of the 60S ribosomal subunit.
- Ministry of Education Key Laboratory of Protein Sciences, Beijing Advanced Innovation Center for Structural Biology, School of Life Sciences, Tsinghua University, Beijing, China.
Organizational Affiliation: