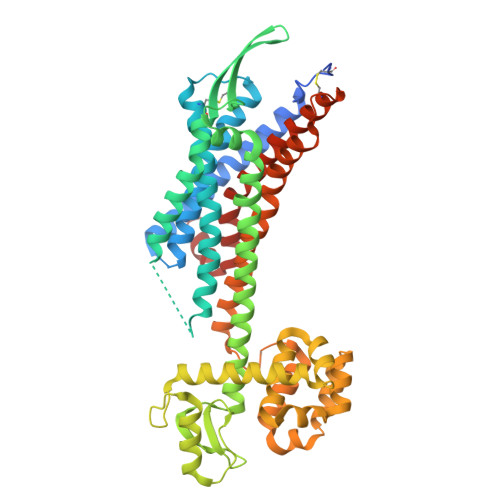
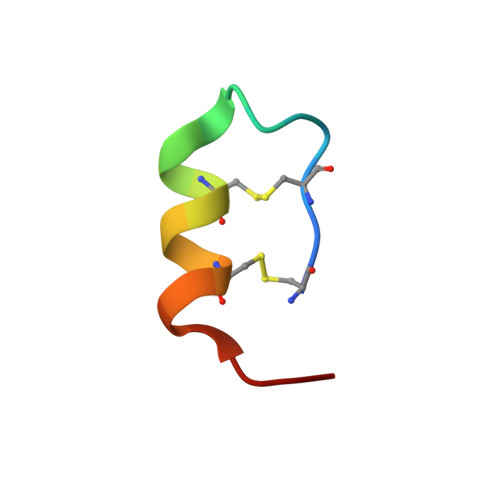
Activation mechanism of endothelin ETB receptor by endothelin-1.
Shihoya, W., Nishizawa, T., Okuta, A., Tani, K., Dohmae, N., Fujiyoshi, Y., Nureki, O., Doi, T.(2016) Nature 537: 363-368
- PubMed: 27595334
- DOI: https://doi.org/10.1038/nature19319
- Primary Citation of Related Structures:
5GLH, 5GLI - PubMed Abstract:
Endothelin, a 21-amino-acid peptide, participates in various physiological processes, such as regulation of vascular tone, humoral homeostasis, neural crest cell development and neurotransmission. Endothelin and its G-protein-coupled receptor are involved in the development of various diseases, such as pulmonary arterial hypertension, and thus are important therapeutic targets. Here we report crystal structures of human endothelin type B receptor in the ligand-free form and in complex with the endogenous agonist endothelin-1. The structures and mutation analysis reveal the mechanism for the isopeptide selectivity between endothelin-1 and -3. Transmembrane helices 1, 2, 6 and 7 move and envelop the entire endothelin peptide, in a virtually irreversible manner. The agonist-induced conformational changes are propagated to the receptor core and the cytoplasmic G-protein coupling interface, and probably induce conformational flexibility in TM6. A comparison with the M2 muscarinic receptor suggests a shared mechanism for signal transduction in class A G-protein-coupled receptors.
- Department of Basic Medicinal Sciences, Graduate School of Pharmaceutical Sciences, Nagoya University, Chikusa, Nagoya 464-8601, Japan.
Organizational Affiliation: