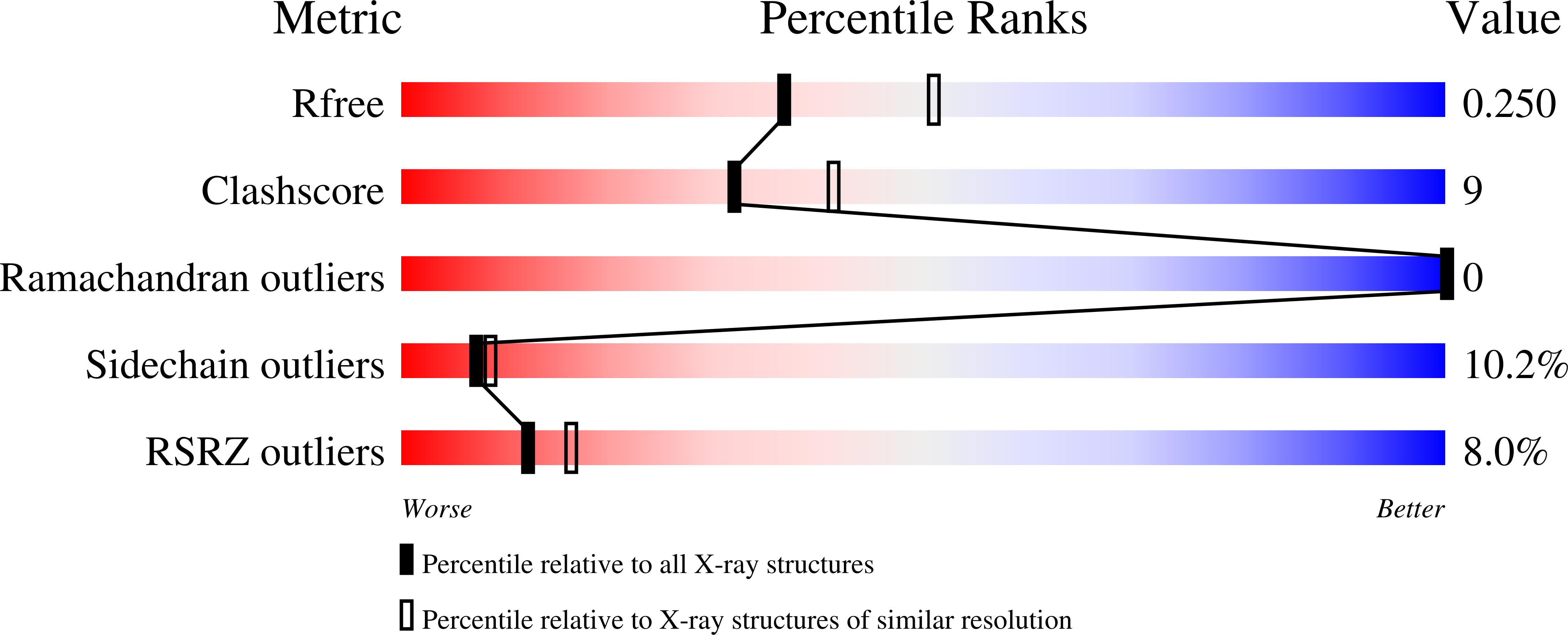
Structural Basis for the Endoribonuclease Activity of the Type III-A Crispr-Associated Protein Csm6.
Niewoehner, O., Jinek, M.(2016) RNA 22: 318
- PubMed: 26763118
- DOI: https://doi.org/10.1261/rna.054098.115
- Primary Citation of Related Structures:
5FSH - PubMed Abstract:
Prokaryotic CRISPR-Cas systems provide an RNA-guided mechanism for genome defense against mobile genetic elements such as viruses and plasmids. In type III-A CRISPR-Cas systems, the RNA-guided multisubunit Csm effector complex targets both single-stranded RNAs and double-stranded DNAs. In addition to the Csm complex, efficient anti-plasmid immunity mediated by type III-A systems also requires the CRISPR-associated protein Csm6. Here we report the crystal structure of Csm6 from Thermus thermophilus and show that the protein is a ssRNA-specific endoribonuclease. The structure reveals a dimeric architecture generated by interactions involving the N-terminal CARF and C-terminal HEPN domains. HEPN domain dimerization leads to the formation of a composite ribonuclease active site. Consistently, mutations of invariant active site residues impair catalytic activity in vitro. We further show that the ribonuclease activity of Csm6 is conserved across orthologs, suggesting that it plays an important functional role in CRISPR-Cas systems. The dimer interface of the CARF domains features a conserved electropositive pocket that may function as a ligand-binding site for allosteric control of ribonuclease activity. Altogether, our work suggests that Csm6 proteins provide an auxiliary RNA-targeting interference mechanism in type III-A CRISPR-Cas systems that operates in conjunction with the RNA- and DNA-targeting endonuclease activities of the Csm effector complex.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Zurich CH-8057, Switzerland.