Kdgf, the Missing Link in the Microbial Metabolism of Uronate Sugars from Pectin and Alginate.
Hobbs, J.K., Lee, S.M., Robb, M., Hof, F., Barr, C., Abe, K.T., Hehemann, J., Mclean, R., Abbott, D.W., Boraston, A.B.(2016) Proc Natl Acad Sci U S A 113: 6188
- PubMed: 27185956
- DOI: https://doi.org/10.1073/pnas.1524214113
- Primary Citation of Related Structures:
5FPX, 5FPZ, 5FQ0 - PubMed Abstract:
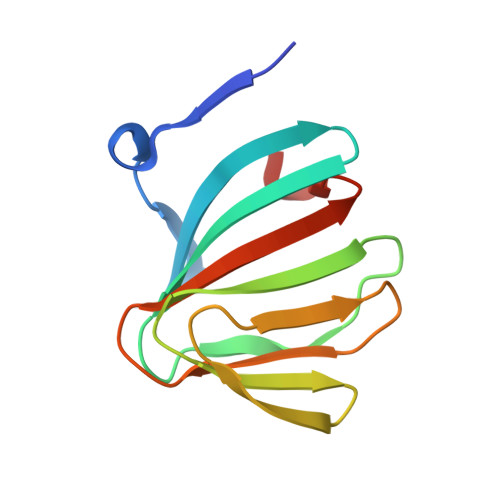
Uronates are charged sugars that form the basis of two abundant sources of biomass-pectin and alginate-found in the cell walls of terrestrial plants and marine algae, respectively. These polysaccharides represent an important source of carbon to those organisms with the machinery to degrade them. The microbial pathways of pectin and alginate metabolism are well studied and essentially parallel; in both cases, unsaturated monouronates are produced and processed into the key metabolite 2-keto-3-deoxygluconate (KDG). The enzymes required to catalyze each step have been identified within pectinolytic and alginolytic microbes; yet the function of a small ORF, kdgF, which cooccurs with the genes for these enzymes, is unknown. Here we show that KdgF catalyzes the conversion of pectin- and alginate-derived 4,5-unsaturated monouronates to linear ketonized forms, a step in uronate metabolism that was previously thought to occur spontaneously. Using enzyme assays, NMR, mutagenesis, and deletion of kdgF, we show that KdgF proteins from both pectinolytic and alginolytic bacteria catalyze the ketonization of unsaturated monouronates and contribute to efficient production of KDG. We also report the X-ray crystal structures of two KdgF proteins and propose a mechanism for catalysis. The discovery of the function of KdgF fills a 50-y-old gap in the knowledge of uronate metabolism. Our findings have implications not only for the understanding of an important metabolic pathway, but also the role of pectinolysis in plant-pathogen virulence and the growing interest in the use of pectin and alginate as feedstocks for biofuel production.
- Department of Biochemistry and Microbiology, University of Victoria, Victoria, BC, V8W 3P6, Canada;
Organizational Affiliation: