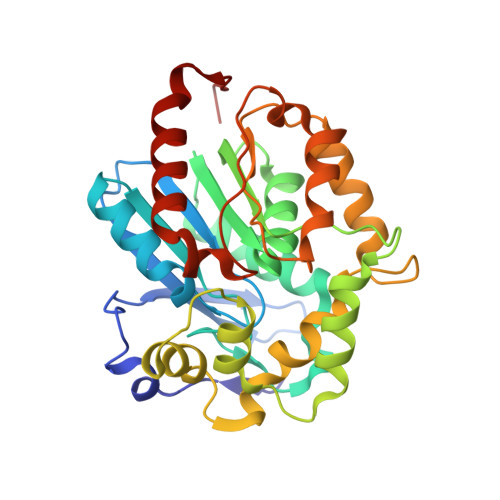
Biochemical characterization of two haloalkane dehalogenases: DccA from Caulobacter crescentus and DsaA from Saccharomonospora azurea.
Carlucci, L., Zhou, E., Malashkevich, V.N., Almo, S.C., Mundorff, E.C.(2016) Protein Sci 25: 877-886
- PubMed: 26833751
- DOI: https://doi.org/10.1002/pro.2895
- Primary Citation of Related Structures:
5ESR - PubMed Abstract:
Two putative haloalkane dehalogenases (HLDs) of the HLD-I subfamily, DccA from Caulobacter crescentus and DsaA from Saccharomonospora azurea, have been identified based on sequence comparisons with functionally characterized HLD enzymes. The two genes were synthesized, functionally expressed in E. coli and shown to have activity toward a panel of haloalkane substrates. DsaA has a moderate activity level and a preference for long (greater than 3 carbons) brominated substrates, but little activity toward chlorinated alkanes. DccA shows high activity with both long brominated and chlorinated alkanes. The structure of DccA was determined by X-ray crystallography and was refined to 1.5 Å resolution. The enzyme has a large and open binding pocket with two well-defined access tunnels. A structural alignment of HLD-I subfamily members suggests a possible basis for substrate specificity is due to access tunnel size.
- Department of Chemistry, Hofstra University, Hempstead, New York, 11549.
Organizational Affiliation: