Structural characterization and practical fluorescence applications of the F45W ubiquitin mutant
Nakasone, M.A., Geng, C., Chojnacki, M., Glickman, M., Paukstelis, P.J., Fushman, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Polyubiquitin-B | 76 | Homo sapiens | Mutation(s): 0 Gene Names: UBB | 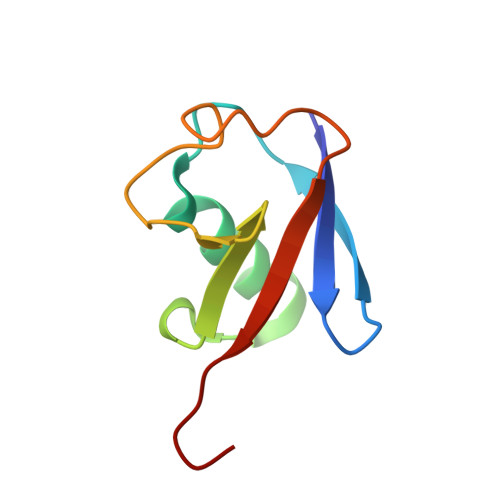 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0CG47 (Homo sapiens) Explore P0CG47 Go to UniProtKB: P0CG47 | |||||
PHAROS: P0CG47 GTEx: ENSG00000170315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0CG47 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Polyubiquitin-B | 76 | Homo sapiens | Mutation(s): 1 Gene Names: UBB | 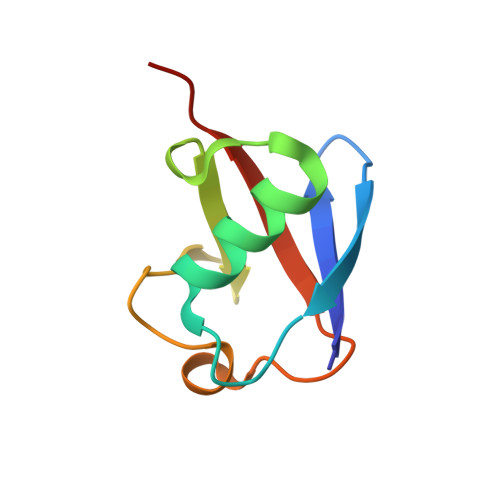 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0CG47 (Homo sapiens) Explore P0CG47 Go to UniProtKB: P0CG47 | |||||
PHAROS: P0CG47 GTEx: ENSG00000170315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0CG47 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | G [auth B], H [auth D], I [auth E], J [auth E] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.87 | α = 90 |
| b = 78.92 | β = 97.93 |
| c = 91.51 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| Aimless | data scaling |
| PHASER | phasing |
| iMOSFLM | data reduction |