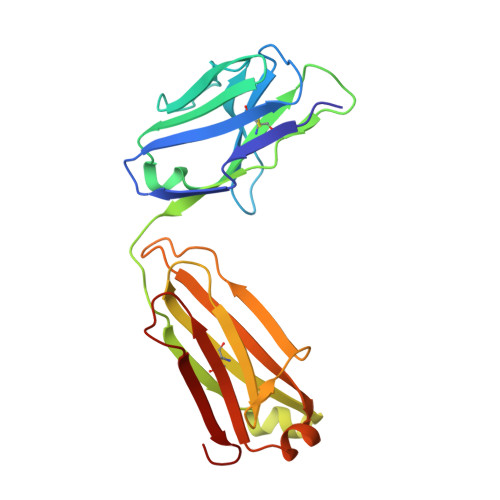
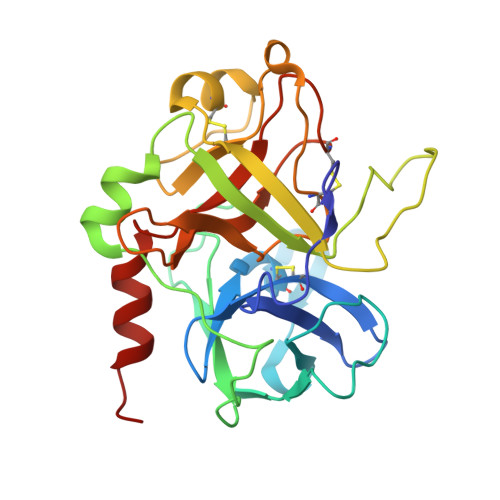
Discovery and characterization of an antibody directed against exosite I of thrombin.
Baglin, T.P., Langdown, J., Frasson, R., Huntington, J.A.(2016) J Thromb Haemost 14: 137-142
- PubMed: 26469093
- DOI: https://doi.org/10.1111/jth.13171
- Primary Citation of Related Structures:
5E8E - PubMed Abstract:
ESSENTIALS: An IgA paraprotein with anti-thrombin activity was not associated with a severe bleeding phenotype. This observation challenges the paradigm that anticoagulant therapy necessarily increases bleeding risk. Characterization of the antibody showed that it specifically binds to thrombin exosite I. A therapeutic drug with the properties of this antibody might be an antithrombotic that doesn't cause bleeding. We report the case of a 54-year-old female who presented with a traumatic subdural hemorrhage. Coagulation tests were markedly prolonged due to the presence of an anti-thrombin IgA paraprotein at 3 g L(-1) . The patient made a complete recovery and has had no abnormal bleeding during a 7-year follow-up, despite the persistence of the paraprotein. To determine how the paraprotein prolonged clotting tests by defining its target and its epitope. The paraprotein was purified and added to normal pooled plasma for in vitro clotting assays. Binding studies were conducted to determine the affinity of the IgA for thrombin. The Fab was isolated and crystallized with thrombin. The purified IgA was sufficient to confer the patient's in vitro coagulation profile in normal pooled plasma, and was found to bind specifically and with high affinity to thrombin. A crystal structure of the Fab fragment in complex with thrombin revealed an exosite I interaction involving CDRH3 of the antibody. Although the patient originally presented with a subdural bleed, the hematoma resolved without intervention, and no other bleeding event occurred during the subsequent 7 years. During this period, the patient's IgA paraprotein levels have remained constant at 3 g L(-1) , suggesting that the presence of a high-affinity, exosite I-directed antibody is consistent with normal hemostasis. A therapeutic derivative of this antibody might therefore permit antithrombotic dose escalation without an associated increase in the risk of bleeding.
- Department of Haematology, Addenbrooke's Hospital, Cambridge University Hospitals NHS Trust, Cambridge, UK.
Organizational Affiliation: