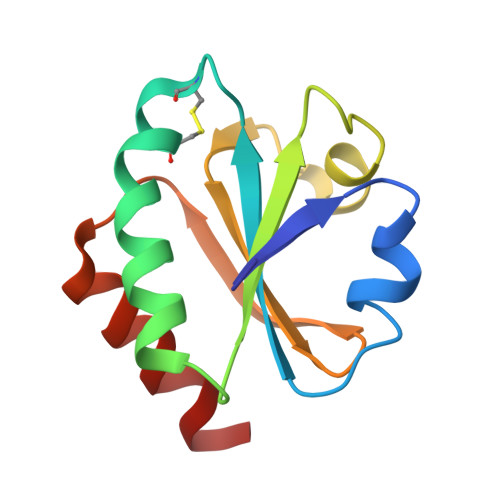
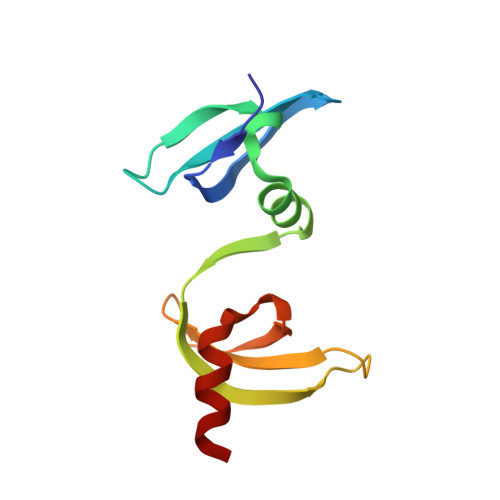
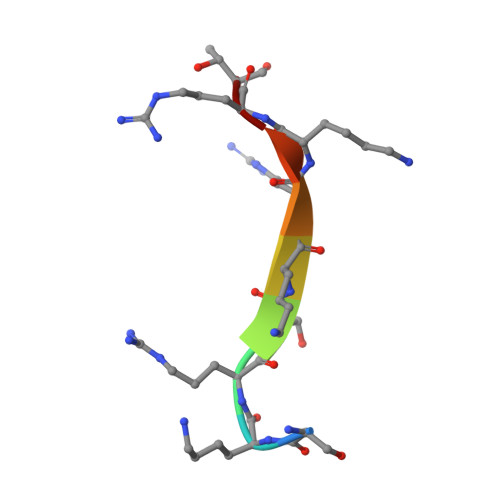
Structural basis for cpSRP43 chromodomain selectivity and dynamics in Alb3 insertase interaction.
Horn, A., Hennig, J., Ahmed, Y.L., Stier, G., Wild, K., Sattler, M., Sinning, I.(2015) Nat Commun 6: 8875-8875
- PubMed: 26568381
- DOI: https://doi.org/10.1038/ncomms9875
- Primary Citation of Related Structures:
2N88, 5E4W, 5E4X - PubMed Abstract:
Canonical membrane protein biogenesis requires co-translational delivery of ribosome-associated proteins to the Sec translocase and depends on the signal recognition particle (SRP) and its receptor (SR). In contrast, high-throughput delivery of abundant light-harvesting chlorophyll a,b-binding proteins (LHCPs) in chloroplasts to the Alb3 insertase occurs post-translationally via a soluble transit complex including the cpSRP43/cpSRP54 heterodimer (cpSRP). Here we describe the molecular mechanisms of tethering cpSRP to the Alb3 insertase by specific interaction of cpSRP43 chromodomain 3 with a linear motif in the Alb3 C-terminal tail. Combining NMR spectroscopy, X-ray crystallography and biochemical analyses, we dissect the structural basis for selectivity of chromodomains 2 and 3 for their respective ligands cpSRP54 and Alb3, respectively. Negative cooperativity in ligand binding can be explained by dynamics in the chromodomain interface. Our study provides a model for membrane recruitment of the transit complex and may serve as a prototype for a functional gain by the tandem arrangement of chromodomains.
- Heidelberg University Biochemistry Center (BZH), INF 328, Heidelberg D-69120, Germany.
Organizational Affiliation: