Crystal structure of IBV papain-like protease PLpro C101S mutant in complex with ubiquitin
Kong, L.Y., Yan, L.M., Zhang, Y., Rao, Z.H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Replicase polyprotein 1ab | 313 | Avian infectious bronchitis virus (strain Beaudette) | Mutation(s): 1 Gene Names: rep, 1a-1b EC: 3.4.22 (PDB Primary Data), 2.7.7.48 (PDB Primary Data), 3.6.4.12 (PDB Primary Data), 3.6.4.13 (PDB Primary Data), 3.1.13 (PDB Primary Data), 3.1 (PDB Primary Data), 2.1.1 (PDB Primary Data) | 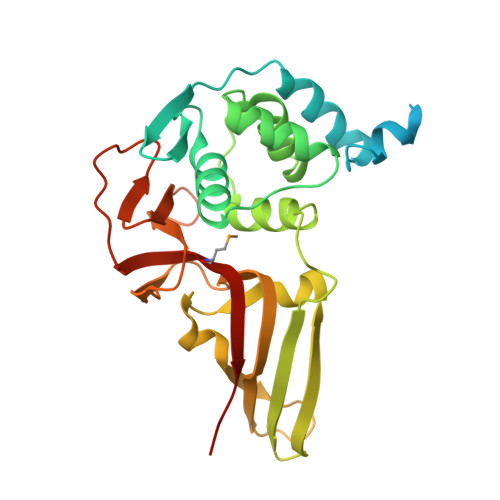 | |
UniProt | |||||
Find proteins for P0C6Y1 (Avian infectious bronchitis virus (strain Beaudette)) Explore P0C6Y1 Go to UniProtKB: P0C6Y1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0C6Y1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-40S ribosomal protein S27a | 76 | Bos taurus | Mutation(s): 0 | 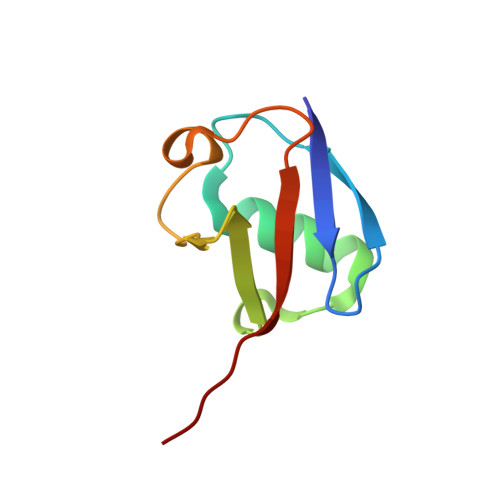 | |
UniProt | |||||
Find proteins for P62992 (Bos taurus) Explore P62992 Go to UniProtKB: P62992 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62992 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | C [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 63.058 | α = 90 |
| b = 74.812 | β = 90 |
| c = 95.502 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data scaling |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China | China | 81330036 and 81322023 |
| National Major Project (973 Program) | China | 2014CB542800 |
| Tsinghua University Initiative Scientific Research Program | China | 20141080146 |