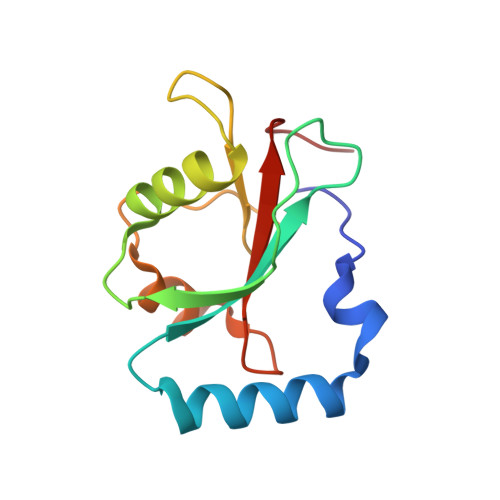
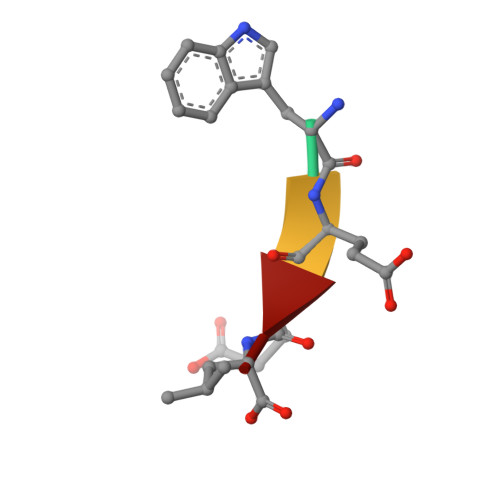
Structural Basis of the Differential Function of the Two C. elegans Atg8 Homologs, LGG-1 and LGG-2, in Autophagy.
Wu, F., Watanabe, Y., Guo, X.Y., Qi, X., Wang, P., Zhao, H.Y., Wang, Z., Fujioka, Y., Zhang, H., Ren, J.Q., Fang, T.C., Shen, Y.X., Feng, W., Hu, J.J., Noda, N.N., Zhang, H.(2015) Mol Cell 60: 914-929
- PubMed: 26687600
- DOI: https://doi.org/10.1016/j.molcel.2015.11.019
- Primary Citation of Related Structures:
5AZF, 5AZG, 5AZH, 5E6N, 5E6O - PubMed Abstract:
Multicellular organisms have multiple homologs of the yeast ATG8 gene, but the differential roles of these homologs in autophagy during development remain largely unknown. Here we investigated structure/function relationships in the two C. elegans Atg8 homologs, LGG-1 and LGG-2. lgg-1 is essential for degradation of protein aggregates, while lgg-2 has cargo-specific and developmental-stage-specific roles in aggregate degradation. Crystallography revealed that the N-terminal tails of LGG-1 and LGG-2 adopt the closed and open form, respectively. LGG-1 and LGG-2 interact differentially with autophagy substrates and Atg proteins, many of which carry a LIR motif. LGG-1 and LGG-2 have structurally distinct substrate binding pockets that prefer different residues in the interacting LIR motif, thus influencing binding specificity. Lipidated LGG-1 and LGG-2 possess distinct membrane tethering and fusion activities, which may result from the N-terminal differences. Our study reveals the differential function of two ATG8 homologs in autophagy during C. elegans development.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, P.R. China.
Organizational Affiliation: