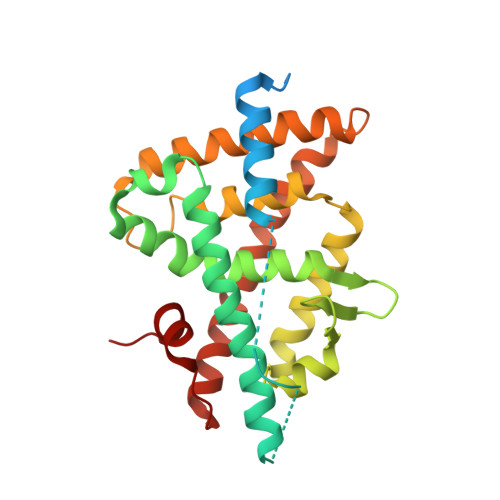
Discovery and structure-guided optimization of tert-butyl 6-(phenoxymethyl)-3-(trifluoromethyl)benzoates as liver X receptor agonists
Matsui, Y., Yamaguchi, T., Yamazaki, T., Yoshida, M., Arai, M., Terasaka, N., Honzumi, S., Wakabayashi, K., Hayashi, S., Nakai, D., Hanzawa, H., Tamaki, K.(2015) Bioorg Med Chem Lett 25: 3914-3920
- PubMed: 26238323
- DOI: https://doi.org/10.1016/j.bmcl.2015.07.047
- Primary Citation of Related Structures:
5AVI, 5AVL - PubMed Abstract:
To obtain potent liver X receptor (LXR) agonists, a structure-activity relationship study was performed on a series of tert-butyl benzoate analogs. As the crystal structure analysis suggested applicable interactions between the LXR ligand-binding domain and the ligands, two key functional groups were introduced. The introduction of the hydroxyl group on the C6-position of the benzoate part enhanced the agonistic activity in a cell-based assay, and the carboxyl group in terminal improved the pharmacokinetic profile in mice, respectively. The obtained compound 32b increased blood ABCA1 mRNA expression without plasma TG elevation in both mice and cynomolgus monkeys.
- Daiichi Sankyo RD Novare Co., Ltd, 1-16-13, Kita-Kasai, Edogawa-ku, Tokyo 134-8630, Japan. Electronic address: matsui.yumi.gk@rdn.daiichisankyo.co.jp.
Organizational Affiliation: