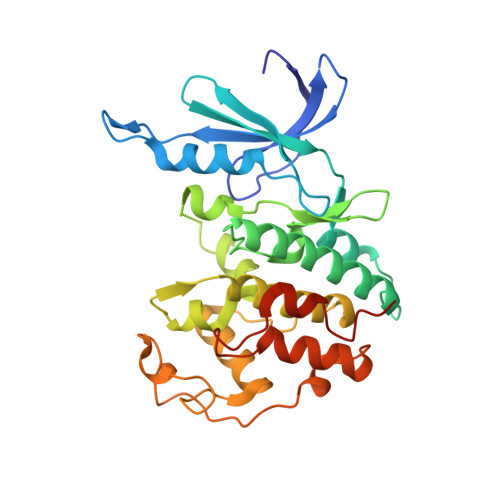
CDK1 structures reveal conserved and unique features of the essential cell cycle CDK.
Brown, N.R., Korolchuk, S., Martin, M.P., Stanley, W.A., Moukhametzianov, R., Noble, M.E., Endicott, J.A.(2015) Nat Commun 6: 6769-6769
- PubMed: 25864384
- DOI: https://doi.org/10.1038/ncomms7769
- Primary Citation of Related Structures:
4Y72, 4YC3, 4YC6, 5HQ0 - PubMed Abstract:
CDK1 is the only essential cell cycle CDK in human cells and is required for successful completion of M-phase. It is the founding member of the CDK family and is conserved across all eukaryotes. Here we report the crystal structures of complexes of CDK1-Cks1 and CDK1-cyclin B-Cks2. These structures confirm the conserved nature of the inactive monomeric CDK fold and its ability to be remodelled by cyclin binding. Relative to CDK2-cyclin A, CDK1-cyclin B is less thermally stable, has a smaller interfacial surface, is more susceptible to activation segment dephosphorylation and shows differences in the substrate sequence features that determine activity. Both CDK1 and CDK2 are potential cancer targets for which selective compounds are required. We also describe the first structure of CDK1 bound to a potent ATP-competitive inhibitor and identify aspects of CDK1 structure and plasticity that might be exploited to develop CDK1-selective inhibitors.
- Department of Biochemistry, University of Oxford, South Parks Road, Oxford, Oxfordshire OX1 3QU, UK.
Organizational Affiliation: