Structural Asymmetry of Phosphodiesterase-9A and a Unique Pocket for Selective Binding of a Potent Enantiomeric Inhibitor.
Huang, M., Shao, Y., Hou, J., Cui, W., Liang, B., Huang, Y., Li, Z., Wu, Y., Zhu, X., Liu, P., Wan, Y., Ke, H., Luo, H.B.(2015) Mol Pharmacol 88: 836-845
- PubMed: 26316540
- DOI: https://doi.org/10.1124/mol.115.099747
- Primary Citation of Related Structures:
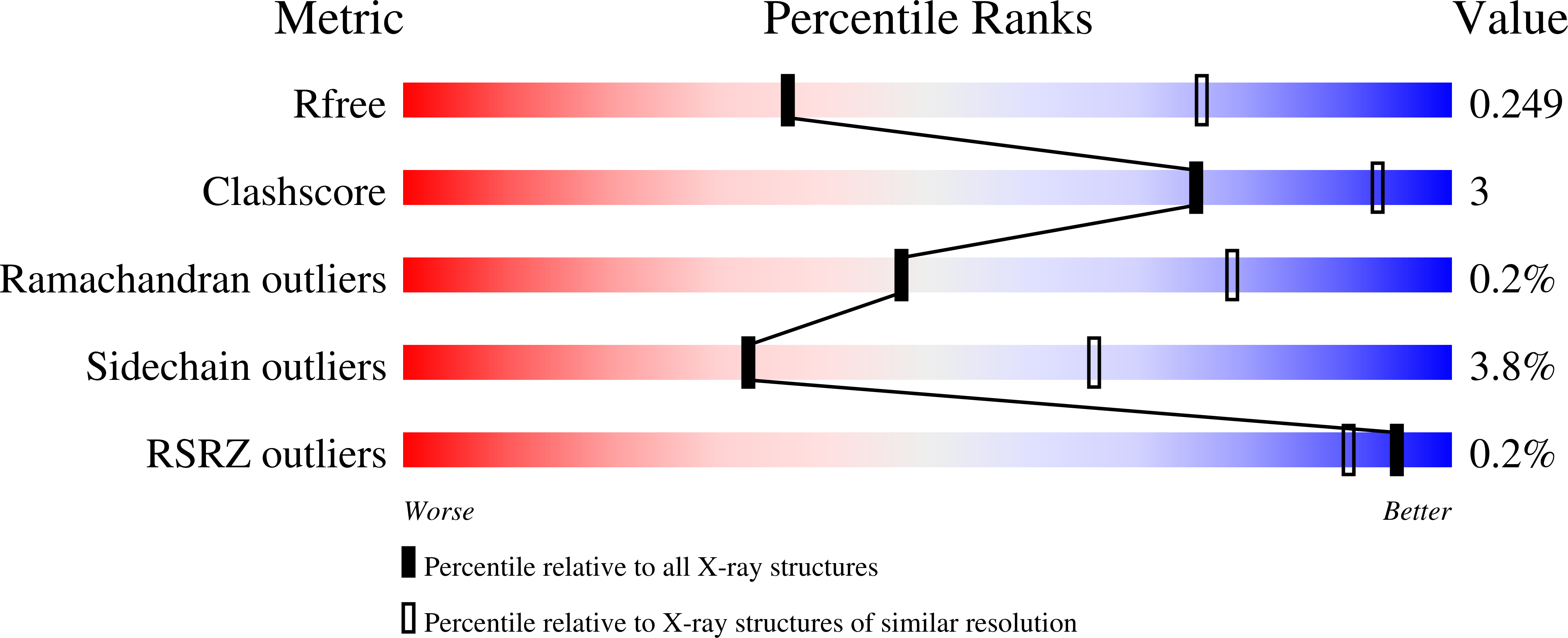
4Y86, 4Y87, 4Y8C - PubMed Abstract:
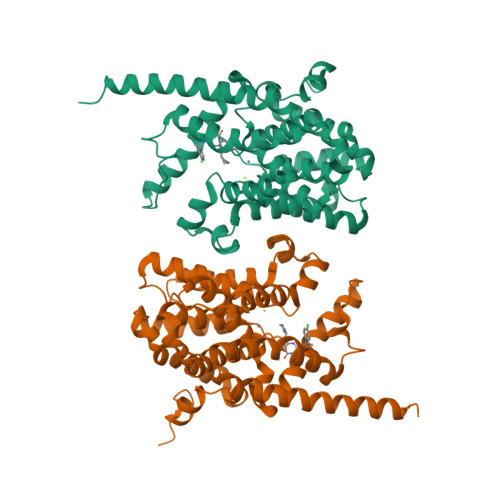
Phosphodiesterase-9 (PDE9) inhibitors have been studied as potential therapeutics for treatment of central nervous system diseases and diabetes. Here, we report the discovery of a new category of PDE9 inhibitors by rational design on the basis of the crystal structures. The best compound, (S)-6-((1-(4-chlorophenyl)ethyl)amino)-1-cyclopentyl-1,5,6,7-tetrahydro-4H-pyrazolo[3,4-day]pyrimidin-4-one [(S)-C33], has an IC50 value of 11 nM against PDE9 and the racemic C33 has bioavailability of 56.5% in the rat pharmacokinetic model. The crystal structures of PDE9 in the complex with racemic C33, (R)-C33, and (S)-C33 reveal subtle conformational asymmetry of two M-loops in the PDE9 dimer and different conformations of two C33 enantiomers. The structures also identified a small hydrophobic pocket that interacts with the tyrosyl tail of (S)-C33 but not with (R)-C33, and is thus possibly useful for improvement of selectivity of PDE9 inhibitors. The asymmetry of the M-loop and the different interactions of the C33 enantiomers imply the necessity to consider the whole PDE9 dimer in the design of inhibitors.
Organizational Affiliation:
School of Chemistry and Chemical Engineering (M.H., J.H., X.Z. Yiq.W.), School of Pharmaceutical Sciences (Y.S., Z.L., Yin.W., P.L., H.-B.L.), Sun Yat-Sen University, Guangzhou, PR China; and Department of Biochemistry and Biophysics and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, North Carolina (W.C., B.L., Y.H., H.K.).