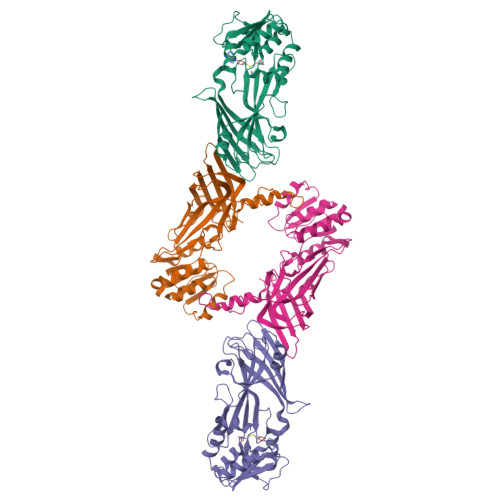
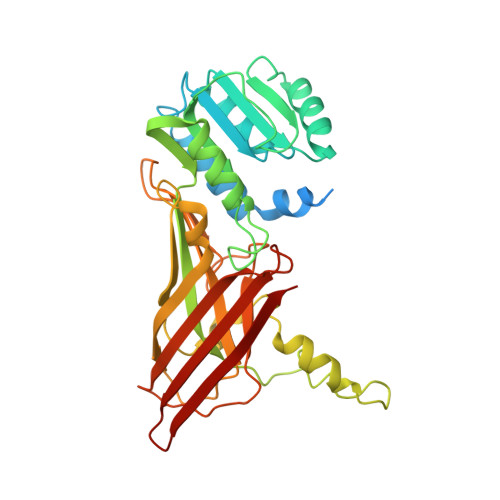
Protein Arginine Methyltransferase 8: Tetrameric Structure and Protein Substrate Specificity
Lee, W.C., Lin, W.L., Matsui, T., Chen, E.S., Wei, T.Y., Lin, W.H., Hu, H., Zheng, Y.G., Tsai, M.D., Ho, M.C.(2015) Biochemistry 54: 7514-7523
- PubMed: 26529540
- DOI: https://doi.org/10.1021/acs.biochem.5b00995
- Primary Citation of Related Structures:
4X41 - PubMed Abstract:
Type I protein arginine methyltransferases (PRMTs) catalyze asymmetric dimethylation of various proteins, and their dysregulations often correlate with tumorigenesis or developmental deficiency. Recent studies have focused on the in vivo substrate identification and the enzyme mechanism with peptide substrates. However, how PRMTs recognize substrates at the protein level remains unknown. PRMT8 is one of the least characterized type I PRMTs, and its crystal structure has not been reported. Here, we report the crystal structure of the PRMT8:SAH complex, identify a new non-histone protein substrate NIFK, and uncover a previously unknown regulatory region specifically required for recognizing NIFK. Instead of the canonical dimeric structure for other type I PRMTs, PRMT8 exists as a tetramer in solution. Using X-ray crystallography in combination with small-angle X-ray scattering experiments, the dimer of dimers architecture in which two PRMT8 dimers are held together mainly by β strand interactions was proposed. Mutation of PRMT8-β15 impedes the methylation of NIFK but still allows methylation of the histone H2A/H2B dimer or a peptide substrate, suggesting a possible structural basis for recognition of protein substrates. Lastly, we observed two PRMT8 dimer orientations resulting in open (without SAH) and closed (with SAH bound) conformations. The comparison between open and closed conformations may provide useful information for PRMT1/8 inhibitor design.
Organizational Affiliation:
Institute of Biological Chemistry, Academia Sinica , Taipei 115, Taiwan.