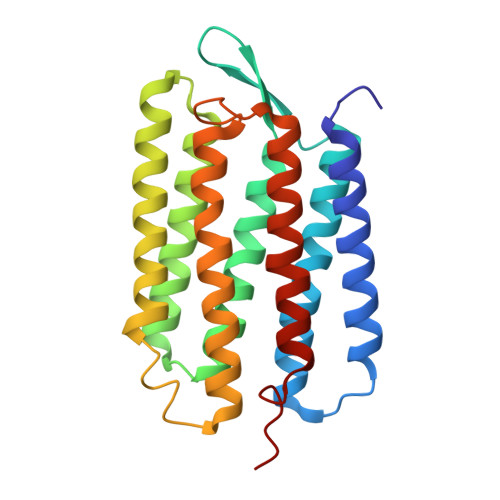
Lipidic cubic phase serial millisecond crystallography using synchrotron radiation.
Nogly, P., James, D., Wang, D., White, T.A., Zatsepin, N., Shilova, A., Nelson, G., Liu, H., Johansson, L., Heymann, M., Jaeger, K., Metz, M., Wickstrand, C., Wu, W., Bath, P., Berntsen, P., Oberthuer, D., Panneels, V., Cherezov, V., Chapman, H., Schertler, G., Neutze, R., Spence, J., Moraes, I., Burghammer, M., Standfuss, J., Weierstall, U.(2015) IUCrJ 2: 168-176
- PubMed: 25866654
- DOI: https://doi.org/10.1107/S2052252514026487
- Primary Citation of Related Structures:
4X31, 4X32 - PubMed Abstract:
Lipidic cubic phases (LCPs) have emerged as successful matrixes for the crystallization of membrane proteins. Moreover, the viscous LCP also provides a highly effective delivery medium for serial femtosecond crystallography (SFX) at X-ray free-electron lasers (XFELs). Here, the adaptation of this technology to perform serial millisecond crystallography (SMX) at more widely available synchrotron microfocus beamlines is described. Compared with conventional microcrystallography, LCP-SMX eliminates the need for difficult handling of individual crystals and allows for data collection at room temperature. The technology is demonstrated by solving a structure of the light-driven proton-pump bacteriorhodopsin (bR) at a resolution of 2.4 Å. The room-temperature structure of bR is very similar to previous cryogenic structures but shows small yet distinct differences in the retinal ligand and proton-transfer pathway.
- Laboratory for Biomolecular Research, Paul Scherrer Institute, Villigen 5232, Switzerland.
Organizational Affiliation: