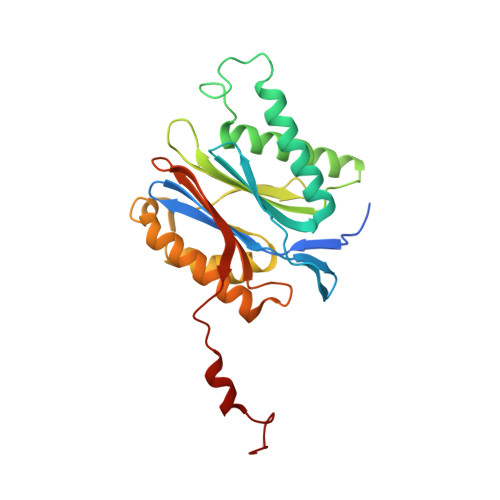
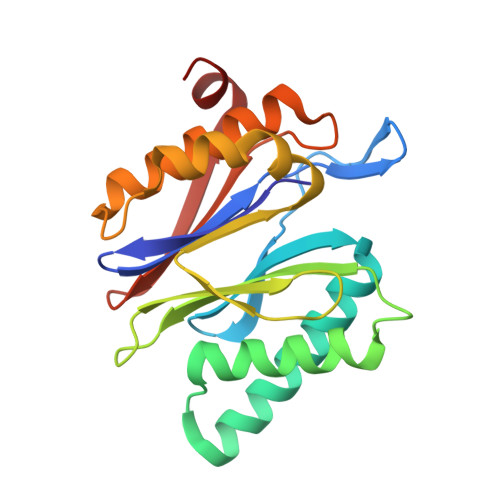
Indolo-Phakellins as beta 5-Specific Noncovalent Proteasome Inhibitors.
Beck, P., Lansdell, T.A., Hewlett, N.M., Tepe, J.J., Groll, M.(2015) Angew Chem Int Ed Engl 54: 2830-2833
- PubMed: 25581903
- DOI: https://doi.org/10.1002/anie.201410168
- Primary Citation of Related Structures:
4RUR - PubMed Abstract:
The proteasome represents an invaluable target for the treatment of cancer and autoimmune disorders. The application of proteasome inhibitors, however, remains limited to blood cancers because their reactive headgroups and peptidic scaffolds convey unfavorable pharmacodynamic properties. Thus, the discovery of more drug-like lead structures is indispensable. In this study, we present the first structure of the proteasome in complex with an indolo-phakellin that exhibits a unique noncovalent binding mode unparalleled by all hitherto reported inhibitors. The natural product inspired pentacyclic alkaloid binds solely and specificially into the spacious S3 subpocket of the proteasomal β5 substrate binding channel, gaining major stabilization through halogen bonding with the protein backbone. The presented compound provides an ideal scaffold for the structure-based design of subunit-specific nonpeptidic proteasome-blockers.
- Center for Integrated Protein Science Munich (CIPSM), Department für Chemie, Technische Universität München, Lichtenbergstrasse 4, 85748 Garching (Germany).
Organizational Affiliation: