Cyrstal structure of O-acetylserine sulfhydrylase ternary complex from Haemophilus influenzae at 2.2 A
Kaushik, A., Ekka, M.K., Singh, A.K., Kumaran, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cysteine synthase | A [auth X] | 333 | Haemophilus influenzae Rd KW20 | Mutation(s): 0 Gene Names: cysK, HI_1103 EC: 2.5.1.47 | 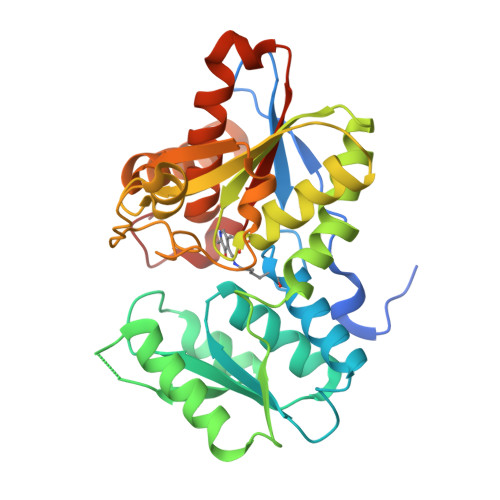 |
UniProt | |||||
Find proteins for P45040 (Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd)) Explore P45040 Go to UniProtKB: P45040 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P45040 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peptide inhibitor | B [auth A] | 8 | N/A | Mutation(s): 0 | 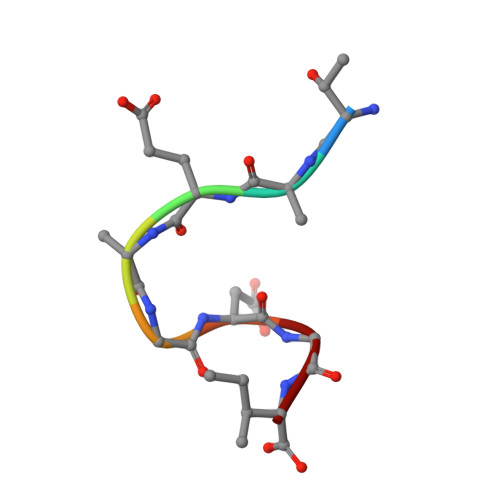 |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| OAS Query on OAS | C [auth A] | O-ACETYLSERINE C5 H9 N O4 VZXPDPZARILFQX-BYPYZUCNSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| LLP Query on LLP | A [auth X] | L-PEPTIDE LINKING | C14 H22 N3 O7 P |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 112.22 | α = 90 |
| b = 112.22 | β = 90 |
| c = 46.03 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHASES | phasing |
| PHENIX | refinement |
| iMOSFLM | data reduction |
| SCALA | data scaling |