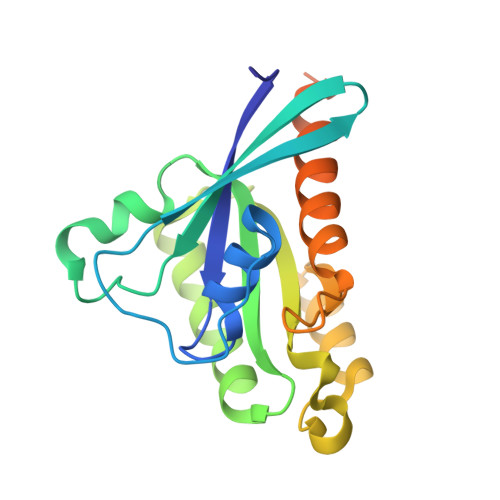
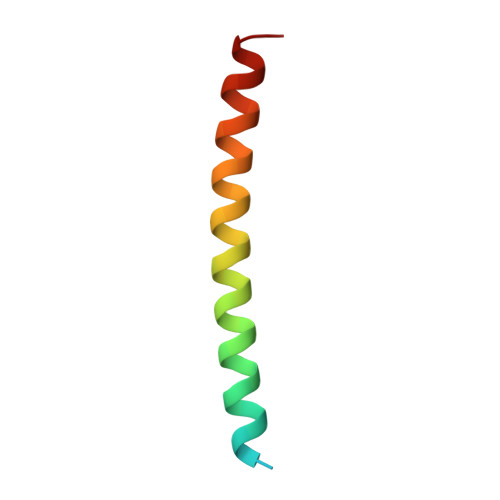
Crystal Structure of the cGMP-dependent Protein Kinase II Leucine Zipper and Rab11b Protein Complex Reveals Molecular Details of G-kinase-specific Interactions.
Reger, A.S., Yang, M.P., Koide-Yoshida, S., Guo, E., Mehta, S., Yuasa, K., Liu, A., Casteel, D.E., Kim, C.(2014) J Biological Chem 289: 25393-25403
- PubMed: 25070890
- DOI: https://doi.org/10.1074/jbc.M114.575894
- Primary Citation of Related Structures:
4OJK - PubMed Abstract:
cGMP-dependent protein kinase (PKG)-interacting proteins (GKIPs) mediate cellular targeting of PKG isoforms by interacting with their leucine zipper (LZ) domains. These interactions prevent aberrant signaling cross-talk between different PKG isotypes. To gain detailed insight into isotype-specific GKIP recognition by PKG, we analyzed the type II PKG leucine zipper domain and found that residues 40-83 dimerized and specifically interacted with Rab11b. Next, we determined a crystal structure of the PKG II LZ-Rab11b complex. The PKG II LZ domain presents a mostly nonpolar surface onto which Rab11b docks, through van der Waals interactions. Contact surfaces in Rab11b are found in switch I and II, interswitch, and the β1/N-terminal regions. This binding surface dramatically differs from that seen in the Rab11 family of interacting protein complex structures. Structural comparison with PKG Iα and Iβ LZs combined with mutagenic analysis reveals that GKIP recognition is mediated through surface charge interactions.
- From the Department of Pharmacology, Baylor College of Medicine, Houston, Texas 77030.
Organizational Affiliation: