Structural and Mechanistic Basis of Zinc Regulation Across the E. coli Zur Regulon.
Gilston, B.A., Wang, S., Marcus, M.D., Canalizo-Hernandez, M.A., Swindell, E.P., Xue, Y., Mondragon, A., O'Halloran, T.V.(2014) PLoS Biol 12: e1001987-e1001987
- PubMed: 25369000
- DOI: https://doi.org/10.1371/journal.pbio.1001987
- Primary Citation of Related Structures:
4MTD, 4MTE - PubMed Abstract:
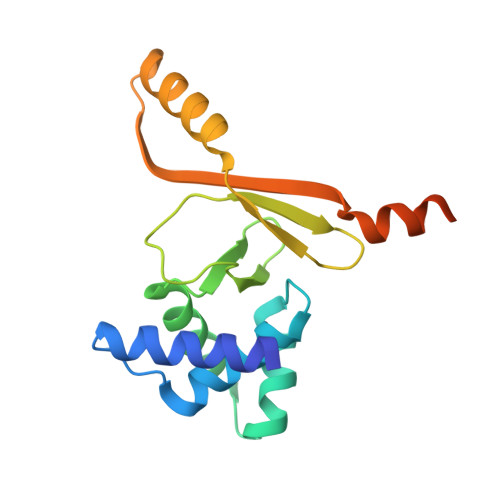
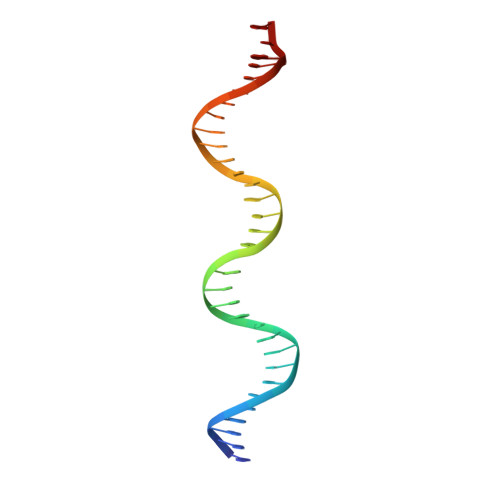
Commensal microbes, whether they are beneficial or pathogenic, are sensitive to host processes that starve or swamp the prokaryote with large fluctuations in local zinc concentration. To understand how microorganisms coordinate a dynamic response to changes in zinc availability at the molecular level, we evaluated the molecular mechanism of the zinc-sensing zinc uptake regulator (Zur) protein at each of the known Zur-regulated genes in Escherichia coli. We solved the structure of zinc-loaded Zur bound to the P(znuABC) promoter and show that this metalloregulatory protein represses gene expression by a highly cooperative binding of two adjacent dimers to essentially encircle the core element of each of the Zur-regulated promoters. Cooperativity in these protein-DNA interactions requires a pair of asymmetric salt bridges between Arg52 and Asp49' that connect otherwise independent dimers. Analysis of the protein-DNA interface led to the discovery of a new member of the Zur-regulon: pliG. We demonstrate this gene is directly regulated by Zur in a zinc responsive manner. The pliG promoter forms stable complexes with either one or two Zur dimers with significantly less protein-DNA cooperativity than observed at other Zur regulon promoters. Comparison of the in vitro Zur-DNA binding affinity at each of four Zur-regulon promoters reveals ca. 10,000-fold variation Zur-DNA binding constants. The degree of Zur repression observed in vivo by comparison of transcript copy number in wild-type and Δzur strains parallels this trend spanning a 100-fold difference. We conclude that the number of ferric uptake regulator (Fur)-family dimers that bind within any given promoter varies significantly and that the thermodynamic profile of the Zur-DNA interactions directly correlates with the physiological response at different promoters.
- Department of Chemistry and The Chemistry of Life Processes Institute, Northwestern University, Evanston, Illinois, United States of America.
Organizational Affiliation: