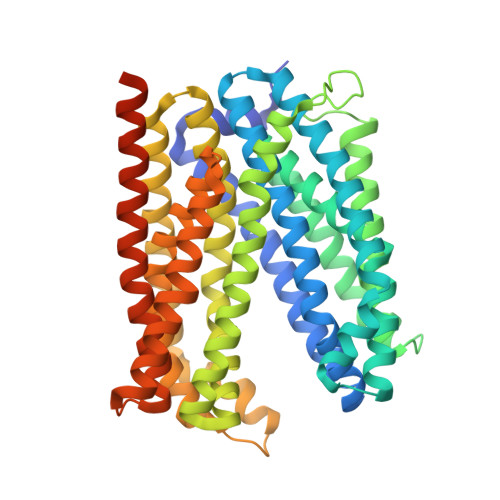
Inward-facing conformation of a multidrug resistance MATE family transporter.
Zakrzewska, S., Mehdipour, A.R., Malviya, V.N., Nonaka, T., Koepke, J., Muenke, C., Hausner, W., Hummer, G., Safarian, S., Michel, H.(2019) Proc Natl Acad Sci U S A 116: 12275-12284
- PubMed: 31160466
- DOI: https://doi.org/10.1073/pnas.1904210116
- Primary Citation of Related Structures:
4MLB, 6FHZ, 6GWH, 6HFB - PubMed Abstract:
Multidrug and toxic compound extrusion (MATE) transporters mediate excretion of xenobiotics and toxic metabolites, thereby conferring multidrug resistance in bacterial pathogens and cancer cells. Structural information on the alternate conformational states and knowledge of the detailed mechanism of MATE transport are of great importance for drug development. However, the structures of MATE transporters are only known in V-shaped outward-facing conformations. Here, we present the crystal structure of a MATE transporter from Pyrococcus furiosus (PfMATE) in the long-sought-after inward-facing state, which was obtained after crystallization in the presence of native lipids. Transition from the outward-facing state to the inward-facing state involves rigid body movements of transmembrane helices (TMs) 2-6 and 8-12 to form an inverted V, facilitated by a loose binding of TM1 and TM7 to their respective bundles and their conformational flexibility. The inward-facing structure of PfMATE in combination with the outward-facing one supports an alternating access mechanism for the MATE family transporters.
- Department of Molecular Membrane Biology, Max Planck Institute of Biophysics, 60438 Frankfurt am Main, Germany.
Organizational Affiliation: