Structural basis of cargo recognitions for class V myosins
Wei, Z., Liu, X., Yu, C., Zhang, M.(2013) Proc Natl Acad Sci U S A 110: 11314-11319
- PubMed: 23798443
- DOI: https://doi.org/10.1073/pnas.1306768110
- Primary Citation of Related Structures:
3WB8, 4KP3 - PubMed Abstract:
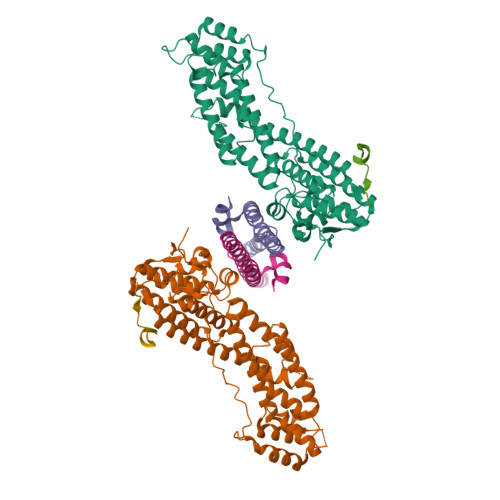
Class V myosins (MyoV), the most studied unconventional myosins, recognize numerous cargos mainly via the motor's globular tail domain (GTD). Little is known regarding how MyoV-GTD recognizes such a diverse array of cargos specifically. Here, we solved the crystal structures of MyoVa-GTD in its apo-form and in complex with two distinct cargos, melanophilin and Rab interacting lysosomal protein-like 2. The apo-MyoVa-GTD structure indicates that most mutations found in patients with Griscelli syndrome, microvillus inclusion disease, or cancers or in "dilute" rodents likely impair the folding of GTD. The MyoVa-GTD/cargo complex structure reveals two distinct cargo-binding surfaces, one primarily via charge-charge interaction and the other mainly via hydrophobic interactions. Structural and biochemical analysis reveal the specific cargo-binding specificities of various isoforms of mammalian MyoV as well as very different cargo recognition mechanisms of MyoV between yeast and higher eukaryotes. The MyoVa-GTD structures resolved here provide a framework for future functional studies of vertebrate class V myosins.
Organizational Affiliation:
Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Kowloon, Hong Kong, China.