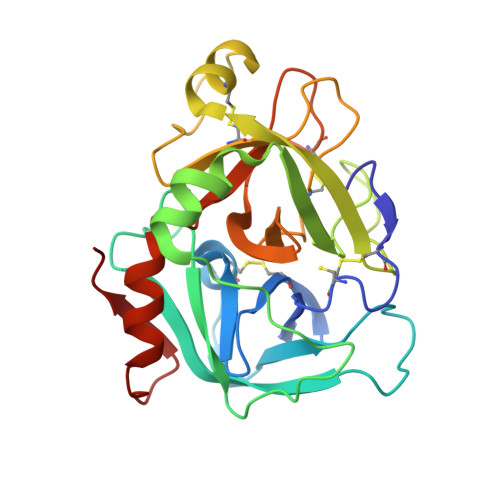
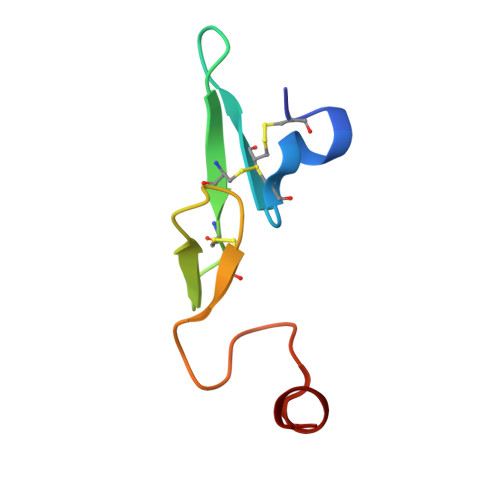
Discovery of nonbenzamidine factor VIIa inhibitors using a biaryl acid scaffold.
Bolton, S.A., Sutton, J.C., Anumula, R., Bisacchi, G.S., Jacobson, B., Slusarchyk, W.A., Treuner, U.D., Wu, S.C., Zhao, G., Pi, Z., Sheriff, S., Smirk, R.A., Bisaha, S., Cheney, D.L., Wei, A., Schumacher, W.A., Hartl, K.S., Liu, E., Zahler, R., Seiler, S.M.(2013) Bioorg Med Chem Lett 23: 5239-5243
- PubMed: 23927973
- DOI: https://doi.org/10.1016/j.bmcl.2013.06.028
- Primary Citation of Related Structures:
4JZD, 4JZE, 4JZF - PubMed Abstract:
In this Letter, we describe the synthesis of several nonamidine analogs of biaryl acid factor VIIa inhibitor 1 containing weakly basic or nonbasic P1 groups. 2-Aminoisoquinoline was found to be an excellent surrogate for the benzamidine group (compound 2) wherein potent inhibition of factor VIIa is maintained relative to most other related serine proteases. In an unanticipated result, the m-benzamide P1 (compounds 21a and 21b) proved to be a viable benzamidine replacement, albeit with a 20-40 fold loss in potency against factor VIIa.
- Bristol-Myers Squibb Research & Development, Hopewell, NJ 08534-5400, USA. scott.bolton@bms.com
Organizational Affiliation: