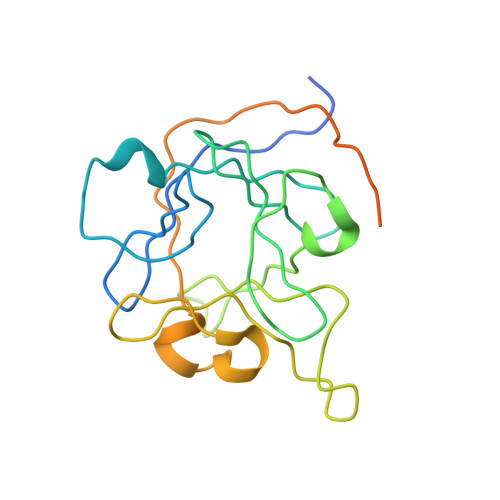
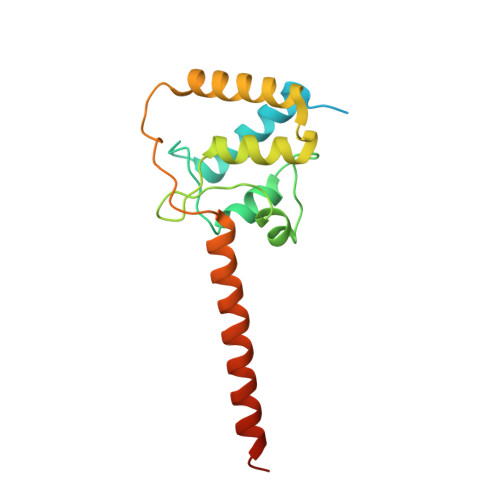
Structural analyses of Ca(2+)/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation.
Wang, C., Chung, B.C., Yan, H., Wang, H.G., Lee, S.Y., Pitt, G.S.(2014) Nat Commun 5: 4896-4896
- PubMed: 25232683
- DOI: https://doi.org/10.1038/ncomms5896
- Primary Citation of Related Structures:
4JPZ, 4JQ0 - PubMed Abstract:
Ca(2+) regulates voltage-gated Na(+) (NaV) channels, and perturbed Ca(2+) regulation of NaV function is associated with epilepsy syndromes, autism and cardiac arrhythmias. Understanding the disease mechanisms, however, has been hindered by a lack of structural information and competing models for how Ca(2+) affects NaV channel function. Here we report the crystal structures of two ternary complexes of a human NaV cytosolic C-terminal domain (CTD), a fibroblast growth factor homologous factor and Ca(2+)/calmodulin (Ca(2+)/CaM). These structures rule out direct binding of Ca(2+) to the NaV CTD and uncover new contacts between CaM and the NaV CTD. Probing these new contacts with biochemical and functional experiments allows us to propose a mechanism by which Ca(2+) could regulate NaV channels. Further, our model provides hints towards understanding the molecular basis of the neurologic disorders and cardiac arrhythmias caused by NaV channel mutations.
- 1] Ion Channel Research Unit, Department of Medicine, Duke University Medical Center, 2 Genome Court, Durham, North Carolina 27710, USA [2] Division of Cardiology, Department of Medicine, Duke University Medical Center, 2 Genome Court, Durham, North Carolina 27710, USA.
Organizational Affiliation: