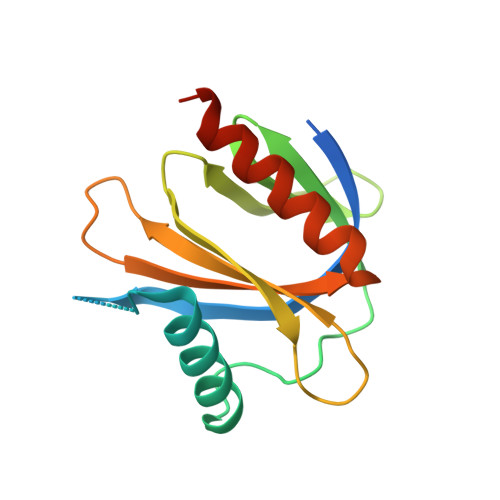
Cocrystal structure of the ICAP1 PTB domain in complex with a KRIT1 peptide.
Liu, W., Boggon, T.J.(2013) Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 494-498
- PubMed: 23695561
- DOI: https://doi.org/10.1107/S1744309113010762
- Primary Citation of Related Structures:
4JIF - PubMed Abstract:
Integrin cytoplasmic domain-associated protein-1 (ICAP1) is a suppressor of integrin activation and directly binds to the cytoplasmic tail of β1 integrins; its binding suppresses integrin activation by competition with talin. Krev/Rap1 interaction trapped-1 (KRIT1) releases ICAP1 suppression of integrin activation by sequestering ICAP1 away from integrin cytoplasmic tails. Here, the cocrystal structure of the PTB domain of ICAP1 in complex with a 29-amino-acid fragment (residues 170-198) of KRIT1 is presented to 1.7 Å resolution [the resolution at which 〈I/σ(I)〉 = 2.9 was 1.83 Å]. In previous studies, the structure of ICAP1 with integrin β1 was determined to 3.0 Å resolution and that of ICAP1 with the N-terminal portion of KRIT1 (residues 1-198) was determined to 2.54 Å resolution; therefore, this study provides the highest resolution structure yet of ICAP1 and allows further detailed analysis of the interaction of ICAP1 with its minimal binding region in KRIT1.
- Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA. liuweizhi@ouc.edu.cn
Organizational Affiliation: