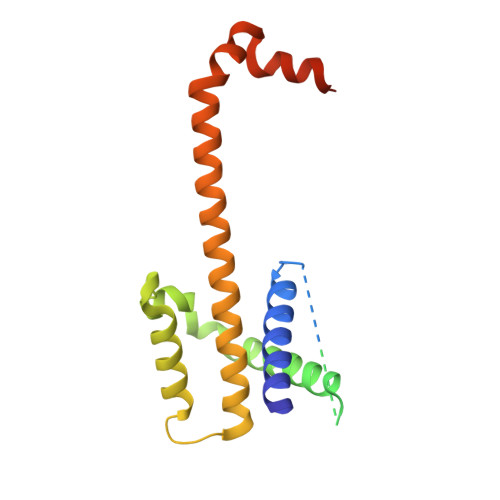
PUMA binding induces partial unfolding within BCL-xL to disrupt p53 binding and promote apoptosis.
Follis, A.V., Chipuk, J.E., Fisher, J.C., Yun, M.K., Grace, C.R., Nourse, A., Baran, K., Ou, L., Min, L., White, S.W., Green, D.R., Kriwacki, R.W.(2013) Nat Chem Biol 9: 163-168
- PubMed: 23340338
- DOI: https://doi.org/10.1038/nchembio.1166
- Primary Citation of Related Structures:
2M03, 2M04, 4HNJ - PubMed Abstract:
Following DNA damage, nuclear p53 induces the expression of PUMA, a BH3-only protein that binds and inhibits the antiapoptotic BCL-2 repertoire, including BCL-xL. PUMA, unique among BH3-only proteins, disrupts the interaction between cytosolic p53 and BCL-xL, allowing p53 to promote apoptosis via direct activation of the BCL-2 effector molecules BAX and BAK. Structural investigations using NMR spectroscopy and X-ray crystallography revealed that PUMA binding induced partial unfolding of two α-helices within BCL-xL. Wild-type PUMA or a PUMA mutant incapable of causing binding-induced unfolding of BCL-xL equivalently inhibited the antiapoptotic BCL-2 repertoire to sensitize for death receptor-activated apoptosis, but only wild-type PUMA promoted p53-dependent, DNA damage-induced apoptosis. Our data suggest that PUMA-induced partial unfolding of BCL-xL disrupts interactions between cytosolic p53 and BCL-xL, releasing the bound p53 to initiate apoptosis. We propose that regulated unfolding of BCL-xL provides a mechanism to promote PUMA-dependent signaling within the apoptotic pathways.
- Department of Structural Biology, St. Jude Children's Research Hospital, Memphis, Tennessee, USA.
Organizational Affiliation: