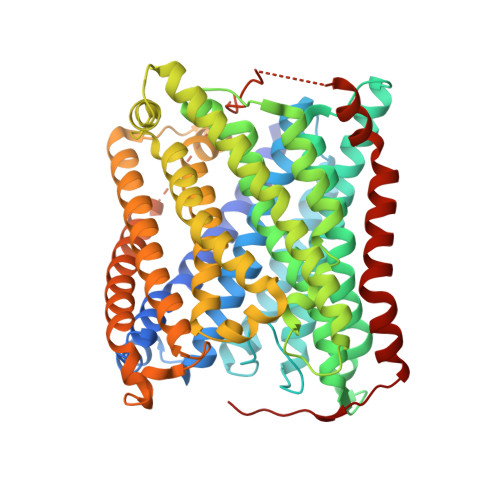
Ligand Access to the Active Site in Thermus thermophilusba(3) and Bovine Heart aa(3) Cytochrome Oxidases.
McDonald, W., Funatogawa, C., Li, Y., Szundi, I., Chen, Y., Fee, J.A., Stout, C.D., Einarsdottir, O.(2013) Biochemistry 52: 640-652
- PubMed: 23282175
- DOI: https://doi.org/10.1021/bi301358a
- Primary Citation of Related Structures:
4GP4, 4GP5, 4GP8 - PubMed Abstract:
Knowledge of the structure and dynamics of the ligand channel(s) in heme-copper oxidases is critical for understanding how the protein environment modulates the functions of these enzymes. Using photolabile NO and O(2) carriers, we recently found that NO and O(2) binding in Thermus thermophilus (Tt) ba(3) is ~10 times faster than in the bovine enzyme, indicating that inherent structural differences affect ligand access in these enzymes. Using X-ray crystallography, time-resolved optical absorption measurements, and theoretical calculations, we investigated ligand access in wild-type Tt ba(3) and the mutants, Y133W, T231F, and Y133W/T231F, in which tyrosine and threonine in the O(2) channel of Tt ba(3) are replaced by the corresponding bulkier tryptophan and phenylalanine, respectively, present in the aa(3) enzymes. NO binding in Y133W and Y133W/T231F was found to be 5 times slower than in wild-type ba(3) and the T231F mutant. The results show that the Tt ba(3) Y133W mutation and the bovine W126 residue physically impede NO access to the binuclear center. In the bovine enzyme, there is a hydrophobic "way station", which may further slow ligand access to the active site. Classical simulations of diffusion of Xe to the active sites in ba(3) and bovine aa(3) show conformational freedom of the bovine F238 and the F231 side chain of the Tt ba(3) Y133W/T231F mutant, with both residues rotating out of the ligand channel, resulting in no effect on ligand access in either enzyme.
- Department of Chemistry and Biochemistry, University of California, Santa Cruz, CA 95064, USA.
Organizational Affiliation: