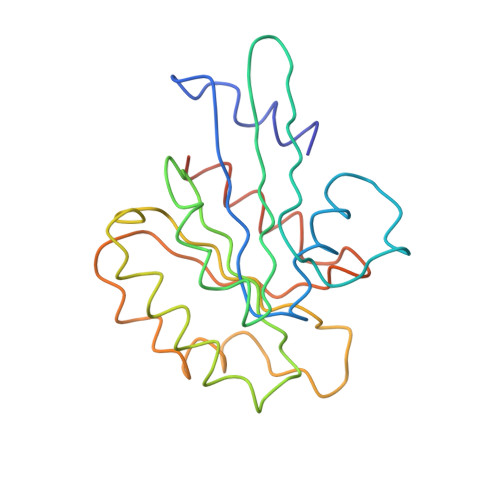
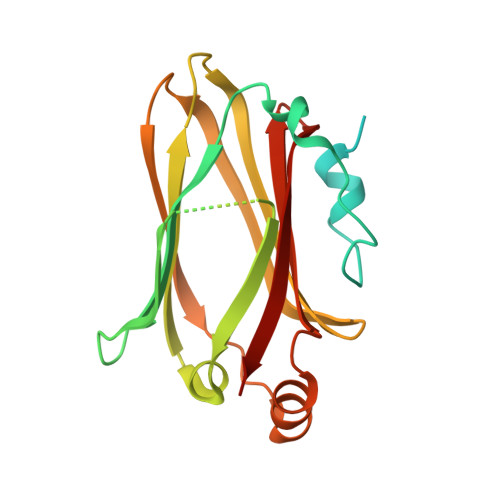
Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119.
Ismail, S.A., Chen, Y.X., Miertzschke, M., Vetter, I.R., Koerner, C., Wittinghofer, A.(2012) EMBO J 31: 4085-4094
- PubMed: 22960633
- DOI: https://doi.org/10.1038/emboj.2012.257
- Primary Citation of Related Structures:
4GOJ, 4GOK - PubMed Abstract:
Access to the ciliary membrane for trans-membrane or membrane-associated proteins is a regulated process. Previously, we have shown that the closely homologous small G proteins Arl2 and Arl3 allosterically regulate prenylated cargo release from PDEδ. UNC119/HRG4 is responsible for ciliary delivery of myristoylated cargo. Here, we show that although Arl3 and Arl2 bind UNC119 with similar affinities, only Arl3 allosterically displaces cargo by accelerating its release by three orders of magnitude. Crystal structures of Arl3 and Arl2 in complex with UNC119a reveal the molecular basis of specificity. Contrary to previous structures of GTP-bound Arf subfamily proteins, the N-terminal amphipathic helix of Arl3·GppNHp is not displaced by the interswitch toggle but remains bound on the surface of the protein. Opposite to the mechanism of cargo release on PDEδ, this induces a widening of the myristoyl binding pocket. This leads us to propose that ciliary targeting of myristoylated proteins is not only dependent on nucleotide status but also on the cellular localization of Arl3.
- Structural Biology Group, Max Planck Institut für Molekulare Physiologie, Dortmund, Germany.
Organizational Affiliation: