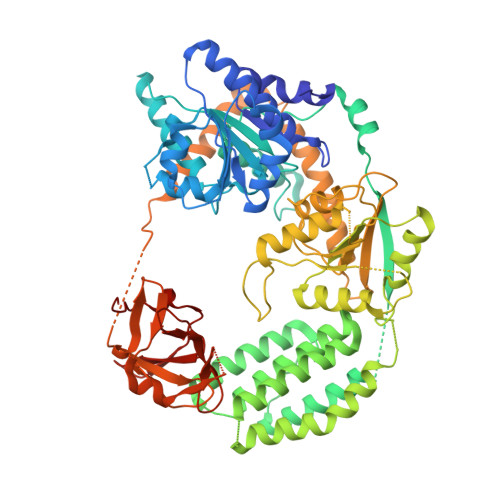
Structural Basis for dsRNA Recognition, Filament Formation, and Antiviral Signal Activation by MDA5.
Wu, B., Peisley, A., Richards, C., Yao, H., Zeng, X., Lin, C., Chu, F., Walz, T., Hur, S.(2013) Cell 152: 276-289
- PubMed: 23273991
- DOI: https://doi.org/10.1016/j.cell.2012.11.048
- Primary Citation of Related Structures:
4GL2 - PubMed Abstract:
MDA5, a viral double-stranded RNA (dsRNA) receptor, shares sequence similarity and signaling pathways with RIG-I yet plays essential functions in antiviral immunity through distinct specificity for viral RNA. Revealing the molecular basis for the functional divergence, we report here the crystal structure of MDA5 bound to dsRNA, which shows how, using the same domain architecture, MDA5 recognizes the internal duplex structure, whereas RIG-I recognizes the terminus of dsRNA. We further show that MDA5 uses direct protein-protein contacts to stack along dsRNA in a head-to-tail arrangement, and that the signaling domain (tandem CARD), which decorates the outside of the core MDA5 filament, also has an intrinsic propensity to oligomerize into an elongated structure that activates the signaling adaptor, MAVS. These data support a model in which MDA5 uses long dsRNA as a signaling platform to cooperatively assemble the core filament, which in turn promotes stochastic assembly of the tandem CARD oligomers for signaling.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: