New 3-amidinophenylalanine-derived inhibitors of matriptase
Hammami, M., Ruehmann, E., Mauerer, M., Heine, A., Guetschow, M., Klebe, G., Steinmetzer, T.(2012) Medchemcomm 3: 807-813
Experimental Data Snapshot
Starting Model: experimental
View more details
(2012) Medchemcomm 3: 807-813
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thrombin light chain | A [auth L], B [auth M] | 36 | Homo sapiens | Mutation(s): 0 EC: 3.4.21.5 | 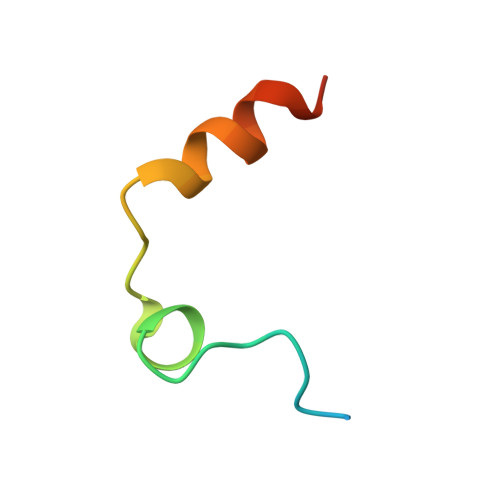 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00734 (Homo sapiens) Explore P00734 Go to UniProtKB: P00734 | |||||
PHAROS: P00734 GTEx: ENSG00000180210 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00734 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thrombin heavy chain | C [auth H], D [auth G] | 259 | Homo sapiens | Mutation(s): 0 EC: 3.4.21.5 | 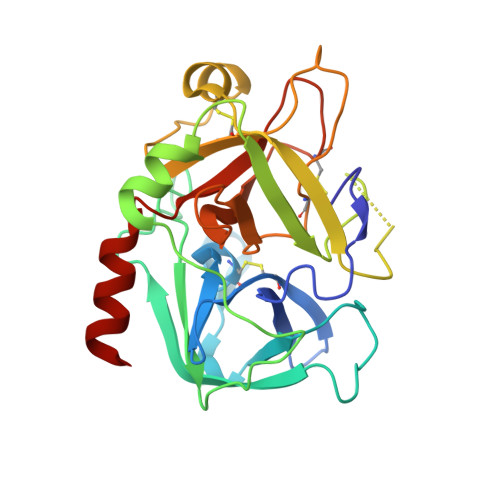 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00734 (Homo sapiens) Explore P00734 Go to UniProtKB: P00734 | |||||
PHAROS: P00734 GTEx: ENSG00000180210 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00734 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P00734-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hirudin variant-2 | E [auth I], F [auth J] | 11 | Hirudo medicinalis | Mutation(s): 0 | 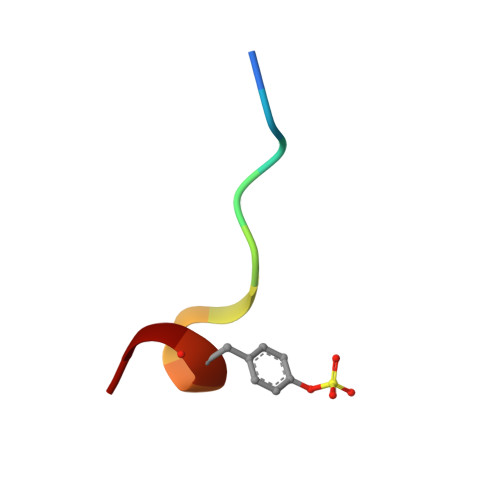 |
UniProt | |||||
Find proteins for P09945 (Hirudo medicinalis) Explore P09945 Go to UniProtKB: P09945 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P09945 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| 0NW Query on 0NW | H, O [auth G] | 3-[(2S)-3-[4-(2-aminoethyl)piperidin-1-yl]-2-{[(2',4'-dichlorobiphenyl-3-yl)sulfonyl]amino}-3-oxopropyl]benzenecarboximidamide C29 H33 Cl2 N5 O3 S ZLCINVZAICQINL-MHZLTWQESA-N |  | ||
| NAG Query on NAG | G [auth H], N [auth G] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| PO4 Query on PO4 | K [auth H], R [auth G] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | L [auth H], M [auth H], S [auth G] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| NA Query on NA | I [auth H], J [auth H], P [auth G], Q [auth G] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TYS Query on TYS | E [auth I], F [auth J] | L-PEPTIDE LINKING | C9 H11 N O6 S |  | TYR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 50.242 | α = 97.61 |
| b = 50.284 | β = 96.61 |
| c = 71.837 | γ = 90.57 |
| Software Name | Purpose |
|---|---|
| MAR345dtb | data collection |
| PHASER | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |