The Variable N-Terminal Region of Ddx5 Contains Structural Elements and Auto-Inhibits its Interaction with Ns5B of Hepatitis C Virus.
Dutta, S., Gupta, G., Choi, Y.W., Kotaka, M., Fielding, B.C., Song, J., Tan, Y.J.(2012) Biochem J 446: 37
- PubMed: 22640416
- DOI: https://doi.org/10.1042/BJ20120001
- Primary Citation of Related Structures:
4A4D - PubMed Abstract:
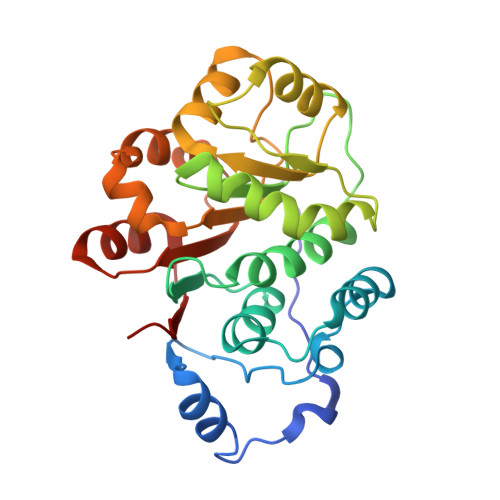
RNA helicases of the DEAD (Asp-Glu-Ala-Asp)-box family of proteins are involved in many aspects of RNA metabolism from transcription to RNA decay, but most of them have also been shown to be multifunctional. The DEAD-box helicase DDX5 of host cells has been shown to interact with the RNA-dependent RNA polymerase (NS5B) of HCV (hepatitis C virus). In the present study, we report the presence of two independent NS5B-binding sites in DDX5, one located at the N-terminus and another at the C-terminus. The N-terminal fragment of DDX5, which consists of the first 305 amino acids and shall be referred as DDX5-N, was expressed and crystallized. The crystal structure shows that domain 1 (residues 79-303) of DDX5 contains the typical features found in the structures of other DEAD-box helicases. DDX5-N also contains the highly variable NTR (N-terminal region) of unknown function and the crystal structure reveals structural elements in part of the NTR, namely residues 52-78. This region forms an extensive loop and an α-helix. From co-immunoprecipitation experiments, the NTR of DDX5-N was observed to auto-inhibit its interaction with NS5B. Interestingly, the α-helix in NTR is essential for this auto-inhibition and seems to mediate the interaction between the highly flexible 1-51 residues in NTR and the NS5B-binding site in DDX5-N. Furthermore, NMR investigations reveal that there is a direct interaction between DDX5 and NS5B in vitro.
Organizational Affiliation:
Institute of Molecular and Cell Biology, A*STAR (Agency for Science, Technology and Research), Singapore 138673.