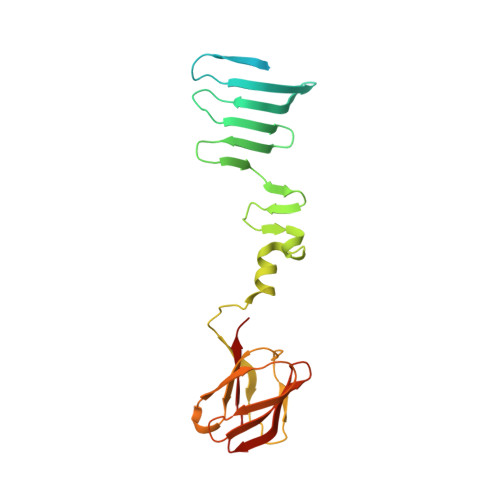
Structure of the receptor-binding carboxy-terminal domain of bacteriophage T7 tail fibers.
Garcia-Doval, C., van Raaij, M.J.(2012) Proc Natl Acad Sci U S A 109: 9390-9395
- PubMed: 22645347
- DOI: https://doi.org/10.1073/pnas.1119719109
- Primary Citation of Related Structures:
4A0T, 4A0U - PubMed Abstract:
The six bacteriophage T7 tail fibers, homo-trimers of gene product 17, are thought to be responsible for the first specific, albeit reversible, attachment to Escherichia coli lipopolysaccharide. The protein trimer forms kinked fibers comprised of an amino-terminal tail-attachment domain, a slender shaft, and a carboxyl-terminal domain composed of several nodules. Previously, we expressed, purified, and crystallized a carboxyl-terminal fragment comprising residues 371-553. Here, we report the structure of this protein trimer, solved using anomalous diffraction and refined at 2 Å resolution. Amino acids 371-447 form a tapered pyramid with a triangular cross-section composed of interlocked β-sheets from each of the three chains. The triangular pyramid domain has three α-helices at its narrow end, which are connected to a carboxyl-terminal three-blade β-propeller tip domain by flexible loops. The monomers of this tip domain each contain an eight-stranded β-sandwich. The exact topology of the β-sandwich fold is novel, but similar to that of knob domains of other viral fibers and the phage Sf6 needle. Several host-range change mutants have been mapped to loops located on the top of this tip domain, suggesting that this surface of the tip domain interacts with receptors on the cell surface.
- Department of Molecular Structure, Centro Nacional de Biotecnologia, Consejo Superior de Investigaciones Cientificas, E-28049 Madrid, Spain.
Organizational Affiliation: