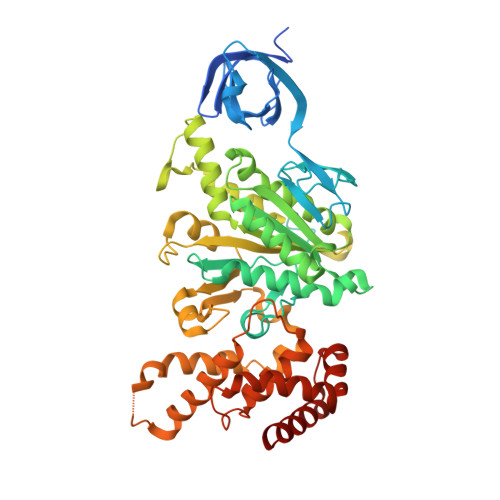
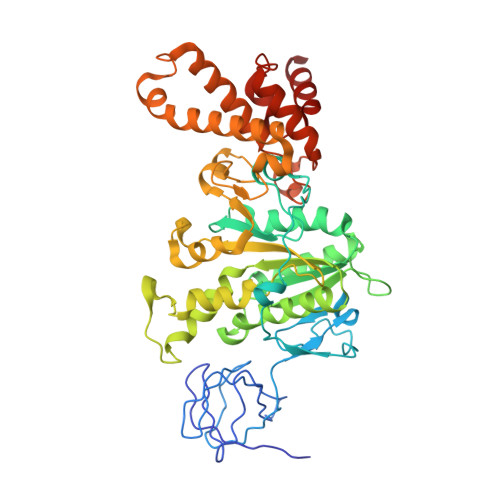
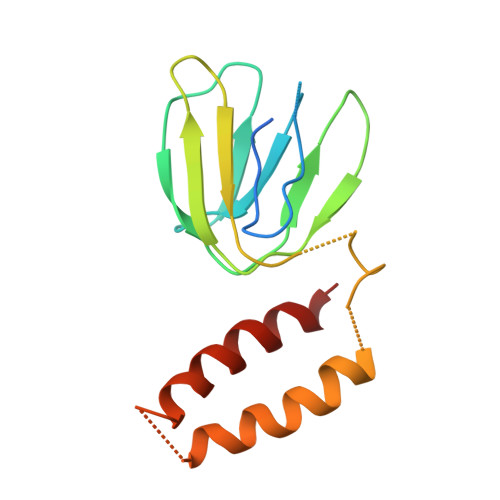
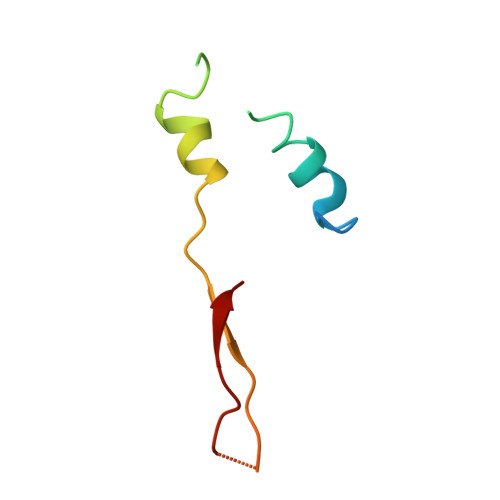
Rotor Architecture in the Yeast and Bovine F(1)-C-Ring Complexes of F-ATP Synthase.
Giraud, M.-F., Paumard, P., Sanchez, C., Brethes, D., Velours, J., Dautant, A.(2012) J Struct Biol 177: 490
- PubMed: 22119846
- DOI: https://doi.org/10.1016/j.jsb.2011.10.015
- Primary Citation of Related Structures:
3ZRY - PubMed Abstract:
The F(1)F(O)-ATP synthase is a rotary molecular nanomotor. F(1) is a chemical motor driven by ATP hydrolysis while F(O) is an electrical motor driven by the proton flow. The two stepping motors are mechanically coupled through a common rotary shaft. Up to now, the three available crystal structures of the F(1)c(10) sub-complex of the yeast F(1)F(O)-ATP synthase were isomorphous and then named yF(1)c(10)(I). In this crystal form, significant interactions of the c(10)-ring with the F(1)-head of neighboring molecules affected the overall conformation of the F(1)-c-ring complex. The symmetry axis of the F(1)-head and the inertia axis of the c-ring were tilted near the interface between the F(1)-central stalk and the c-ring rotor, resulting in an unbalanced machine. We have solved a new crystal form of the F(1)c(10) complex, named yF(1)c(10)(II), inhibited by adenylyl-imidodiphosphate (AMP-PNP) and dicyclohexylcarbodiimide (DCCD), at 6.5Å resolution in which the crystal packing has a weaker influence over the conformation of the F(1)-c-ring complex. yF(1)c(10)(II) provides a model of a more efficient generator. yF(1)c(10)(II) and bovine bF(1)c(8) structures share a common rotor architecture with the inertia center of the F(1)-stator close to the rotor axis.
- Univ. de Bordeaux, IBGC, UMR 5095, F-33000 Bordeaux, France. marie-france.giraud@ibgc.cnrs.fr
Organizational Affiliation: