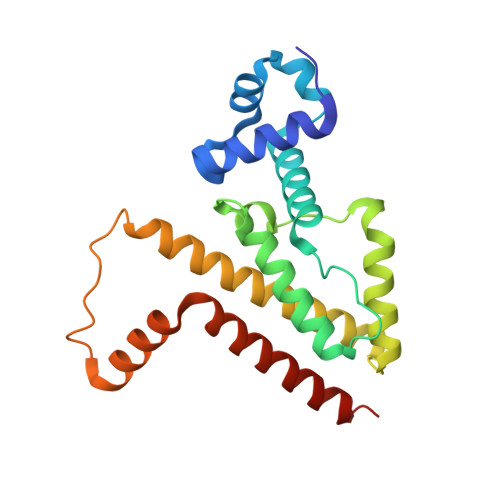
An Exclusive Alpha/Beta Code Directs Allostery in Tetr-Peptide Complexes.
Sevvana, M., Goetz, C., Goeke, D., Wimmer, C., Berens, C., Hillen, W., Muller, Y.A.(2012) J Mol Biology 416: 46
- PubMed: 22178479
- DOI: https://doi.org/10.1016/j.jmb.2011.12.008
- Primary Citation of Related Structures:
3ZQF, 3ZQG, 3ZQH, 3ZQI - PubMed Abstract:
The allosteric mechanism of one of the best characterized bacterial transcription regulators, tetracycline repressor (TetR), has recently been questioned. Tetracycline binding induces cooperative folding of TetR, as suggested by recent unfolding studies, rather than switching between two defined conformational states, namely a DNA-binding-competent conformation and a non-DNA-binding conformation. Upon ligand binding, a host of near-native multiconformational structures collapse into a single, highly stabilized protein conformation that is no longer able to bind DNA. Here, structure-function studies performed with four synthetic peptides that bind to TetR and mimic the function of low-molecular-weight effectors, such as tetracyclines, provide new means to discriminate between different allosteric models. Whereas two inducing peptides bind in an extended β-like conformation, two anti-inducing peptides form an α-helix in the effector binding site of TetR. This exclusive bimodal interaction mode coincides with two distinct overall conformations of TetR, namely one that is identical with induced TetR and one that mirrors the DNA-bound state of TetR. Urea-induced unfolding studies show no increase in thermodynamic stability for any of the peptide complexes, although fluorescence measurements demonstrate peptide binding to TetR. This strongly suggests that, at least for these peptide effectors, a classical two-state allosteric model best describes TetR function.
- Lehrstuhl für Biotechnik, Department of Biology, Friedrich-Alexander University Erlangen-Nuremberg, Henkestr. 91, D-91052 Erlangen, Germany.
Organizational Affiliation: