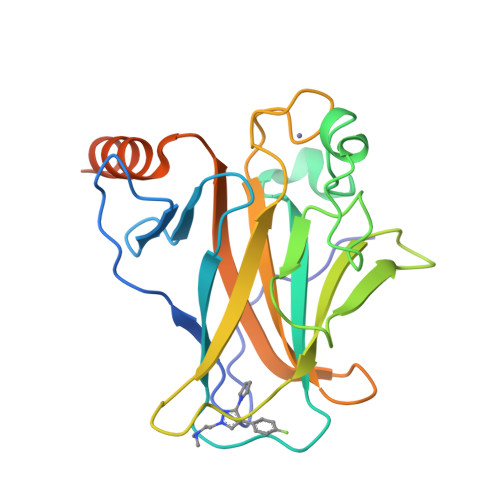
Small molecule induced reactivation of mutant p53 in cancer cells.
Liu, X., Wilcken, R., Joerger, A.C., Chuckowree, I.S., Amin, J., Spencer, J., Fersht, A.R.(2013) Nucleic Acids Res 41: 6034-6044
- PubMed: 23630318
- DOI: https://doi.org/10.1093/nar/gkt305
- Primary Citation of Related Structures:
3ZME - PubMed Abstract:
The p53 cancer mutant Y220C is an excellent paradigm for rescuing the function of conformationally unstable p53 mutants because it has a unique surface crevice that can be targeted by small-molecule stabilizers. Here, we have identified a compound, PK7088, which is active in vitro: PK7088 bound to the mutant with a dissociation constant of 140 μM and raised its melting temperature, and we have determined the binding mode of a close structural analogue by X-ray crystallography. We showed that PK7088 is biologically active in cancer cells carrying the Y220C mutant by a battery of tests. PK7088 increased the amount of folded mutant protein with wild-type conformation, as monitored by immunofluorescence, and restored its transcriptional functions. It induced p53-Y220C-dependent growth inhibition, cell-cycle arrest and apoptosis. Most notably, PK7088 increased the expression levels of p21 and the proapoptotic NOXA protein. PK7088 worked synergistically with Nutlin-3 on up-regulating p21 expression, whereas Nutlin-3 on its own had no effect, consistent with its mechanism of action. PK7088 also restored non-transcriptional apoptotic functions of p53 by triggering nuclear export of BAX to the mitochondria. We suggest a set of criteria for assigning activation of p53.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge CB2 0QH, UK.