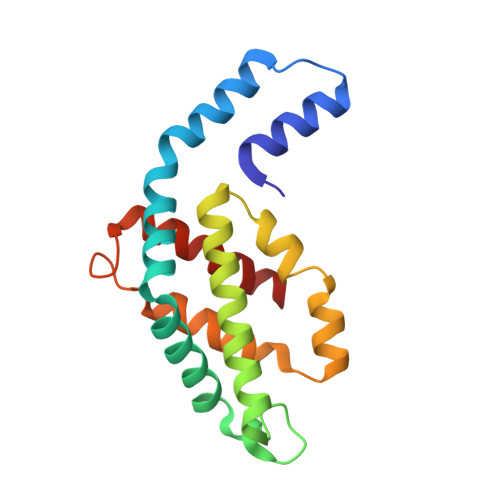
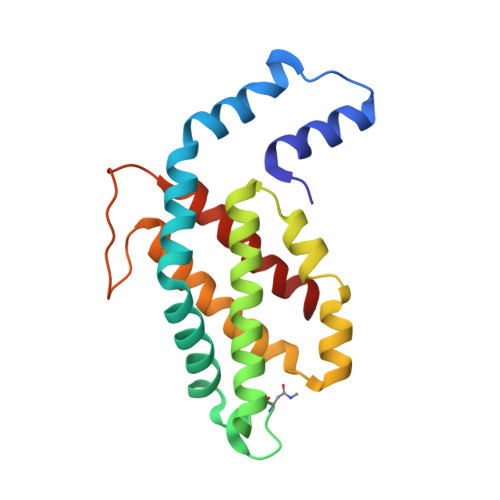
pH-dependent structural conformations of B-phycoerythrin from Porphyridium cruentum
Camara-Artigas, A., Bacarizo, J., Andujar-Sanchez, M., Ortiz-Salmeron, E., Mesa-Valle, C., Cuadri, C., Martin-Garcia, J.M., Martinez-Rodriguez, S., Mazzuca-Sobczuk, T., Ibanez, M.J., Allen, J.P.(2012) FEBS J 279: 3680-3691
- PubMed: 22863205
- DOI: https://doi.org/10.1111/j.1742-4658.2012.08730.x
- Primary Citation of Related Structures:
3V57, 3V58 - PubMed Abstract:
B-phycoerythrin from the red alga Porphyridium cruentum was crystallized using the technique of capillary counter-diffusion. Crystals belonging to the space group R3 with almost identical unit cell constants and diffracting to 1.85 and 1.70 Å were obtained at pH values of 5 and 8, respectively. The most important difference between structures is the presence of the residue His88α in two different conformations at pH 8. This residue is placed next to the chromophore phycoerythrobilin PEB82α and the new conformation results in the relocation of the hydrogen-bond network and hydration around PEB82α, which probably contributes to the observed pH dependence of the optical spectrum associated with this chromophore. Comparison with the structures of B-phycoerythrin from other red algae shows differences in the conformation of the A-ring of the chromophore PEB139α. This conformational difference in B-phycoerythrin from P. cruentum enables the formation of several hydrogen bonds that connect PEB139α with the chromophore PEB158β at the (αβ)(3) hexamer association interface. The possible influence of these structural differences on the optical spectrum and the ability of the protein to perform energy transfer are discussed, with the two pH-dependent conformations of His88α and PEB82α being proposed as representing critical structural features that are correlated with the pH dependence of the optical spectrum and transient optical states during energy transfer.
- Department of Physical Chemistry, Biochemistry and Inorganic Chemistry, Agrifood Campus of International Excellence (CeiA3), University of Almería, Spain.
Organizational Affiliation: