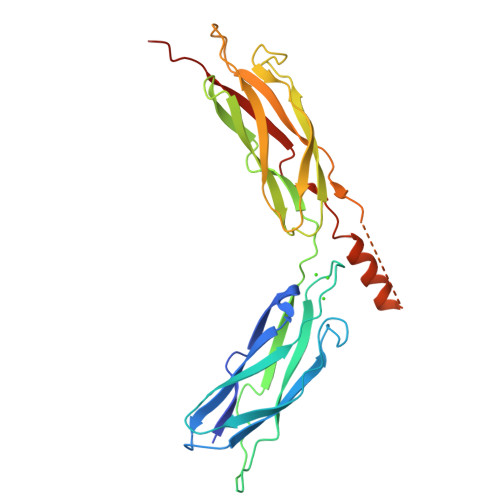
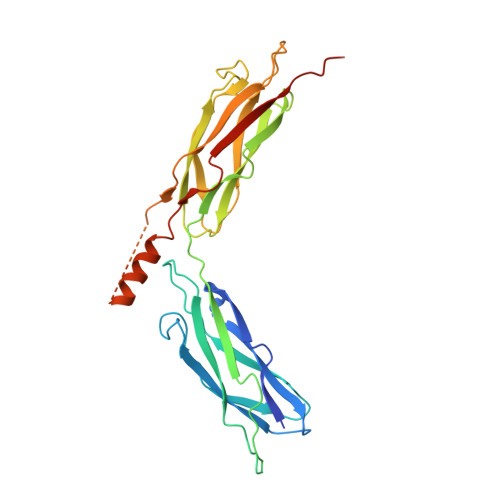
A common Ca2+-driven interdomain module governs eukaryotic NCX regulation.
Giladi, M., Sasson, Y., Fang, X., Hiller, R., Buki, T., Wang, Y.X., Hirsch, J.A., Khananshvili, D.(2012) PLoS One 7: e39985-e39985
- PubMed: 22768191
- DOI: https://doi.org/10.1371/journal.pone.0039985
- Primary Citation of Related Structures:
3US9 - PubMed Abstract:
Na(+)/Ca(2+) exchanger (NCX) proteins mediate Ca(2+)-fluxes across the cell membrane to maintain Ca(2+) homeostasis in many cell types. Eukaryotic NCX contains Ca(2+)-binding regulatory domains, CBD1 and CBD2. Ca(2+) binding to a primary sensor (Ca3-Ca4 sites) on CBD1 activates mammalian NCXs, whereas CALX, a Drosophila NCX ortholog, displays an inhibitory response to regulatory Ca(2+). To further elucidate the underlying regulatory mechanisms, we determined the 2.7 Å crystal structure of mammalian CBD12-E454K, a two-domain construct that retains wild-type properties. In conjunction with stopped-flow kinetics and SAXS (small-angle X-ray scattering) analyses of CBD12 mutants, we show that Ca(2+) binding to Ca3-Ca4 sites tethers the domains via a network of interdomain salt-bridges. This Ca(2+)-driven interdomain switch controls slow dissociation of "occluded" Ca(2+) from the primary sensor and thus dictates Ca(2+) sensing dynamics. In the Ca(2+)-bound conformation, the interdomain angle of CBD12 is very similar in NCX and CALX, meaning that the interdomain distances cannot account for regulatory diversity in NCX and CALX. Since the two-domain interface is nearly identical among eukaryotic NCXs, including CALX, we suggest that the Ca(2+)-driven interdomain switch described here represents a general mechanism for initial conduction of regulatory signals in NCX variants.
Organizational Affiliation:
Department of Physiology and Pharmacology, Sackler School of Medicine, Tel-Aviv University, Ramat-Aviv, Tel-Aviv, Israel.