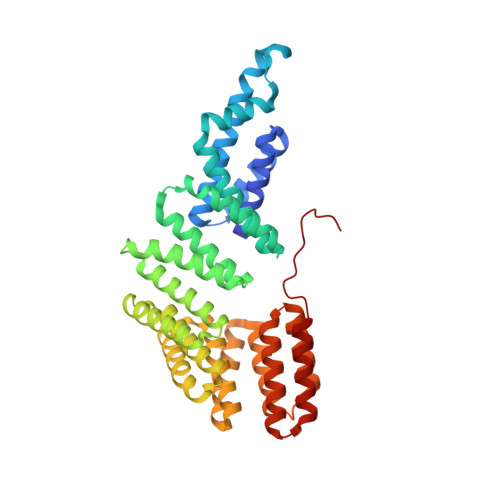
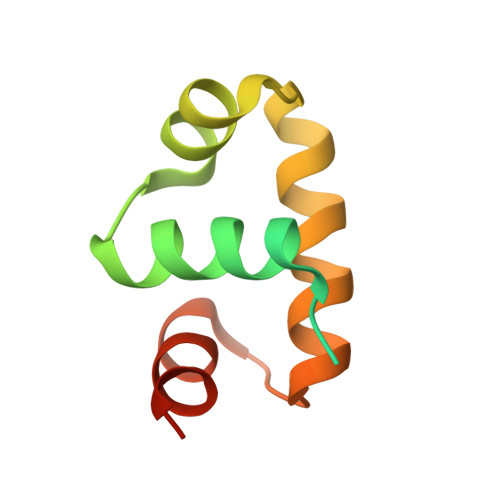
Structural basis of response regulator inhibition by a bacterial anti-activator protein.
Baker, M.D., Neiditch, M.B.(2011) PLoS Biol 9: e1001226-e1001226
- PubMed: 22215984
- DOI: https://doi.org/10.1371/journal.pbio.1001226
- Primary Citation of Related Structures:
3ULQ - PubMed Abstract:
The complex interplay between the response regulator ComA, the anti-activator RapF, and the signaling peptide PhrF controls competence development in Bacillus subtilis. More specifically, ComA drives the expression of genetic competence genes, while RapF inhibits the interaction of ComA with its target promoters. The signaling peptide PhrF accumulates at high cell density and upregulates genetic competence by antagonizing the interaction of RapF and ComA. How RapF functions mechanistically to inhibit ComA activity and how PhrF in turn antagonizes the RapF-ComA interaction were unknown. Here we present the X-ray crystal structure of RapF in complex with the ComA DNA binding domain. Along with biochemical and genetic studies, the X-ray crystal structure reveals how RapF mechanistically regulates ComA function. Interestingly, we found that a RapF surface mimics DNA to block ComA binding to its target promoters. Furthermore, RapF is a monomer either alone or in complex with PhrF, and it undergoes a conformational change upon binding to PhrF, which likely causes the dissociation of ComA from the RapF-ComA complex. Finally, we compare the structure of RapF complexed with the ComA DNA binding domain and the structure of RapH complexed with Spo0F. This comparison reveals that RapF and RapH have strikingly similar overall structures, and that they have evolved different, non-overlapping surfaces to interact with diverse cellular targets. To our knowledge, the data presented here reveal the first atomic level insight into the inhibition of response regulator DNA binding by an anti-activator. Compounds that affect the interaction of Rap and Rap-like proteins with their target domains could serve to regulate medically and commercially important phenotypes in numerous Bacillus species, such as sporulation in B. anthracis and sporulation and the production of Cry protein endotoxin in B. thuringiensis.
- Department of Microbiology and Molecular Genetics, UMDNJ-New Jersey Medical School, Newark, New Jersey, United States of America.
Organizational Affiliation: