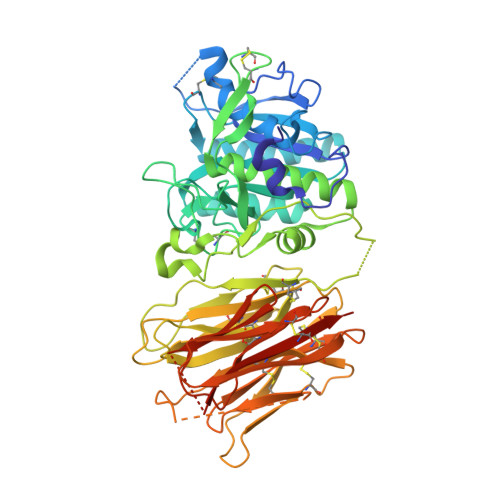
Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates.
Liang, H., Chaparro-Riggers, J., Strop, P., Geng, T., Sutton, J.E., Tsai, D., Bai, L., Abdiche, Y., Dilley, J., Yu, J., Wu, S., Chin, S.M., Lee, N.A., Rossi, A., Lin, J.C., Rajpal, A., Pons, J., Shelton, D.L.(2012) J Pharmacol Exp Ther 340: 228-236
- PubMed: 22019884
- DOI: https://doi.org/10.1124/jpet.111.187419
- Primary Citation of Related Structures:
3SQO - PubMed Abstract:
Proprotein convertase substilisin/kexin type 9 (PCSK9) promotes the degradation of low-density lipoprotein (LDL) receptor (LDLR) and thereby increases serum LDL-cholesterol (LDL-C). We have developed a humanized monoclonal antibody that recognizes the LDLR binding domain of PCSK9. This antibody, J16, and its precursor mouse antibody, J10, potently inhibit PCSK9 binding to the LDLR extracellular domain and PCSK9-mediated down-regulation of LDLR in vitro. In vivo, J10 effectively reduces serum cholesterol in C57BL/6 mice fed normal chow. J16 reduces LDL-C in healthy and diet-induced hypercholesterolemic cynomologous monkeys, but does not significantly affect high-density lipoprotein-cholesterol. Furthermore, J16 greatly lowered LDL-C in hypercholesterolemic monkeys treated with the HMG-CoA reductase inhibitor simvastatin. Our data demonstrate that anti-PCSK9 antibody is a promising LDL-C-lowering agent that is both efficacious and potentially additive to current therapies.
- Rinat Laboratories, Pfizer Inc., 230 East Grand Avenue, South San Francisco, CA 94080, USA. hong.liang@pfizer.com
Organizational Affiliation: