Structural and biochemical characterization of two binding sites for nucleation-promoting factor WASp-VCA on Arp2/3 complex.
Ti, S.C., Jurgenson, C.T., Nolen, B.J., Pollard, T.D.(2011) Proc Natl Acad Sci U S A 108: E463-E471
- PubMed: 21676862
- DOI: https://doi.org/10.1073/pnas.1100125108
- Primary Citation of Related Structures:
3RSE - PubMed Abstract:
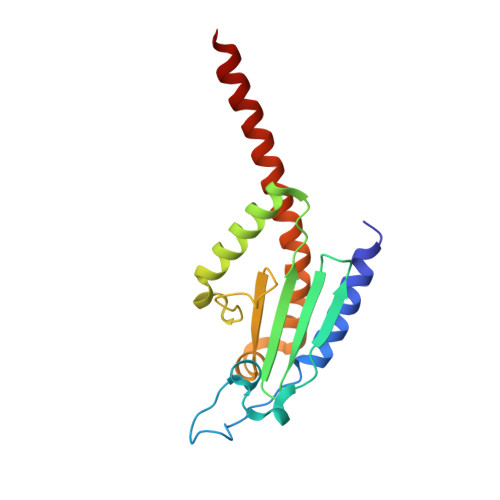
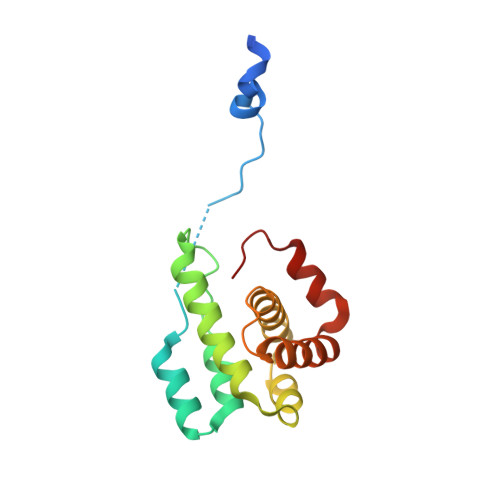
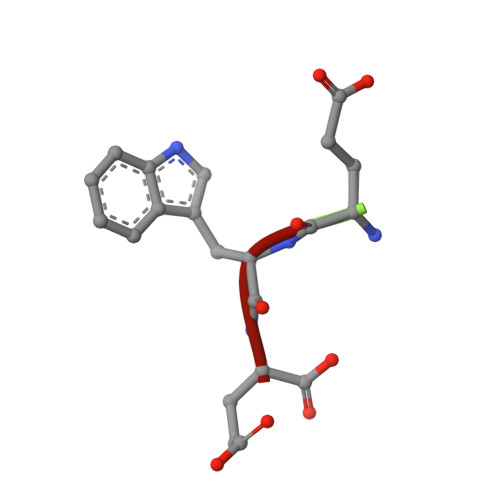
Actin-related protein (Arp) 2/3 complex mediates the formation of actin filament branches during endocytosis and at the leading edge of motile cells. The pathway of branch formation is ambiguous owing to uncertainty regarding the stoichiometry and location of VCA binding sites on Arp2/3 complex. Isothermal titration calorimetry showed that the CA motif from the C terminus of fission yeast WASP (Wsp1p) bound to fission yeast and bovine Arp2/3 complex with a stoichiometry of 2 to 1 and very different affinities for the two sites (K(d)s of 0.13 and 1.6 μM for fission yeast Arp2/3 complex). Equilibrium binding, kinetic, and cross-linking experiments showed that (i) CA at high-affinity site 1 inhibited Arp2/3 complex binding to actin filaments, (ii) low-affinity site 2 had a higher affinity for CA when Arp2/3 complex was bound to actin filaments, and (iii) Arp2/3 complex had a much higher affinity for free CA than VCA cross-linked to an actin monomer. Crystal structures showed the C terminus of CA bound to the low-affinity site 2 on Arp3 of bovine Arp2/3 complex. The C helix is likely to bind to the barbed end groove of Arp3 in a position for VCA to deliver the first actin subunit to the daughter filament.
- Department of Molecular, Cellular, and Developmental Biology, Yale University, New Haven, CT 06520-8103, USA.
Organizational Affiliation: