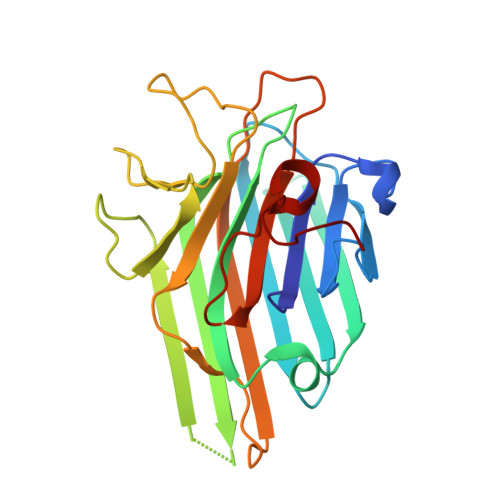
Structure of Dioclea virgata lectin: Relations between carbohydrate binding site and nitric oxide production.
Batista da Nobrega, R., Rocha, B.A., Gadelha, C.A., Santi-Gadelha, T., Pires, A.F., Assreuy, A.M., Nascimento, K.S., Nagano, C.S., Sampaio, A.H., Cavada, B.S., Delatorre, P.(2012) Biochimie 94: 900-906
- PubMed: 22198239
- DOI: https://doi.org/10.1016/j.biochi.2011.12.009
- Primary Citation of Related Structures:
3RRD, 3RS6 - PubMed Abstract:
The lectin of Dioclea virgata (DvirL), both native and complexed with X-man, was submitted to X-ray diffraction analysis and the crystal structure was compared to that of other Diocleinae lectins in order to better understand differences in biological properties, especially with regard to the ability of lectins to induce nitric oxide (NO) production. An association was observed between the volume of the carbohydrate recognition domain (CRD), the ability to induce NO production and the relative positions of Tyr12, Arg228 and Leu99. Thus, differences in biological activity induced by Diocleinae lectins are related to the configuration of amino acid residues in the carbohydrate binding site and to the structural conformation of subsequent regions capable of influencing site-ligand interactions. In conclusion, the ability of Diocleinae lectins to induce NO production depends on CRD configuration.
- Departamento de Biologia Molecular, Universidade Federal da Paraíba, campus l, s/n, 58059-900 João Pessoa, Paraíba, Brazil.
Organizational Affiliation: