Crystal Structure and Characterization of Particulate Methane Monooxygenase from Methylocystis species Strain M.
Smith, S.M., Rawat, S., Telser, J., Hoffman, B.M., Stemmler, T.L., Rosenzweig, A.C.(2011) Biochemistry 50: 10231-10240
- PubMed: 22013879
- DOI: https://doi.org/10.1021/bi200801z
- Primary Citation of Related Structures:
3RFR, 3RGB - PubMed Abstract:
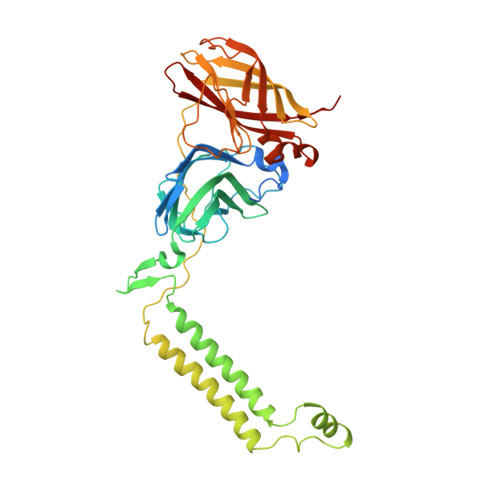
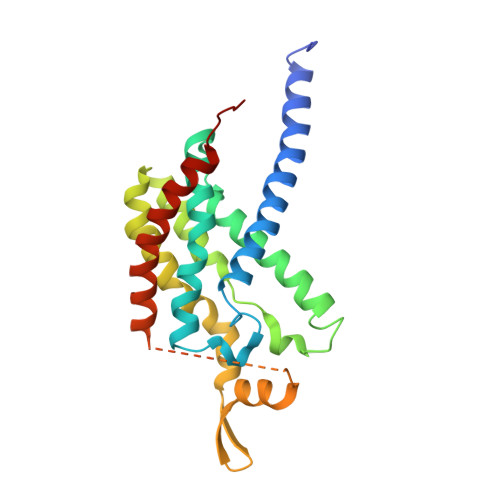
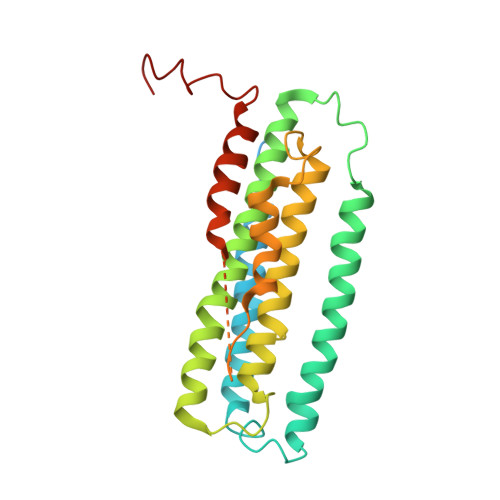
Particulate methane monooxygenase (pMMO) is an integral membrane metalloenzyme that oxidizes methane to methanol in methanotrophic bacteria. Previous biochemical and structural studies of pMMO have focused on preparations from Methylococcus capsulatus (Bath) and Methylosinus trichosporium OB3b. A pMMO from a third organism, Methylocystis species strain M, has been isolated and characterized. Both membrane-bound and solubilized Methylocystis sp. strain M pMMO contain ~2 copper ions per 100 kDa protomer and exhibit copper-dependent propylene epoxidation activity. Spectroscopic data indicate that Methylocystis sp. strain M pMMO contains a mixture of Cu(I) and Cu(II), of which the latter exhibits two distinct type 2 Cu(II) electron paramagnetic resonance (EPR) signals. Extended X-ray absorption fine structure (EXAFS) data are best fit with a mixture of Cu-O/N and Cu-Cu ligand environments with a Cu-Cu interaction at 2.52-2.64 Å. The crystal structure of Methylocystis sp. strain M pMMO was determined to 2.68 Å resolution and is the best quality pMMO structure obtained to date. It provides a revised model for the pmoA and pmoC subunits and has led to an improved model of M. capsulatus (Bath) pMMO. In these new structures, the intramembrane zinc/copper binding site has a different coordination environment from that in previous models.
- Departments of Molecular Biosciences and of Chemistry, Northwestern University, Evanston, Illinois 60208, United States.
Organizational Affiliation: