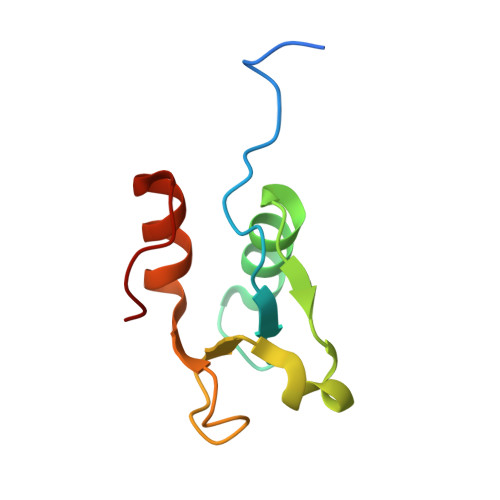
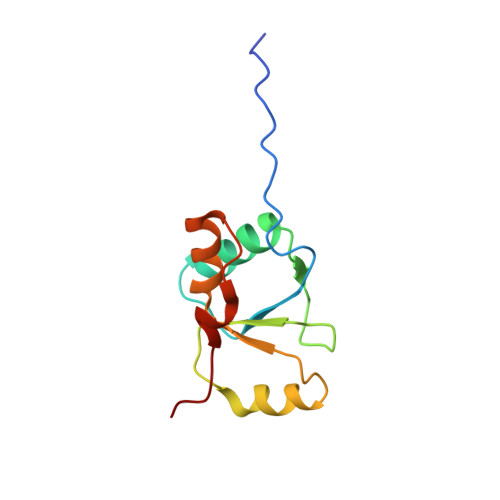
The structural basis for partitioning of the XRCC1/DNA ligase III-{alpha} BRCT-mediated dimer complexes.
Cuneo, M.J., Gabel, S.A., Krahn, J.M., Ricker, M.A., London, R.E.(2011) Nucleic Acids Res 39: 7816-7827
- PubMed: 21652643
- DOI: https://doi.org/10.1093/nar/gkr419
- Primary Citation of Related Structures:
3PC6, 3PC7, 3PC8, 3QVG - PubMed Abstract:
The ultimate step common to almost all DNA repair pathways is the ligation of the nicked intermediate to form contiguous double-stranded DNA. In the mammalian nucleotide and base excision repair pathways, the ligation step is carried out by ligase III-α. For efficient ligation, ligase III-α is constitutively bound to the scaffolding protein XRCC1 through interactions between the C-terminal BRCT domains of each protein. Although structural data for the individual domains has been available, no structure of the complex has been determined and several alternative proposals for this interaction have been advanced. Interpretation of the models is complicated by the formation of homodimers that, depending on the model, may either contribute to, or compete with heterodimer formation. We report here the structures of both homodimer complexes as well as the heterodimer complex. Structural characterization of the heterodimer formed from a longer XRCC1 BRCT domain construct, including residues comprising the interdomain linker region, revealed an expanded heterodimer interface with the ligase III-α BRCT domain. This enhanced linker-mediated binding interface plays a significant role in the determination of heterodimer/homodimer selectivity. These data provide fundamental insights into the structural basis of BRCT-mediated dimerization, and resolve questions related to the organization of this important repair complex.
- National Institute of Environmental Health Sciences, NIH, DHHS, Research Triangle Park, NC, USA.
Organizational Affiliation: