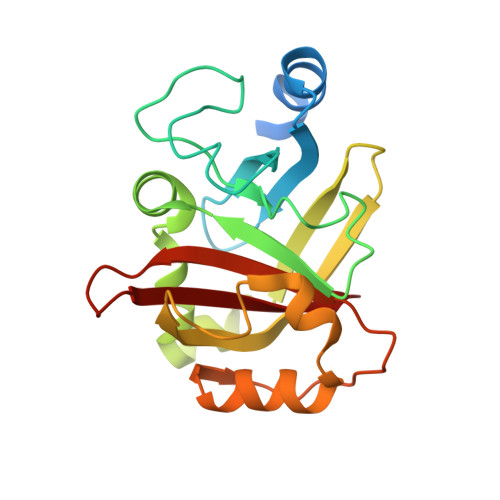
Crystal structure of Spy0129, a Streptococcus pyogenes class B sortase involved in pilus assembly
Kang, H.J., Coulibaly, F., Proft, T., Baker, E.N.(2011) PLoS One 6: e15969-e15969
- PubMed: 21264317
- DOI: https://doi.org/10.1371/journal.pone.0015969
- Primary Citation of Related Structures:
3PSQ - PubMed Abstract:
Sortase enzymes are cysteine transpeptidases that mediate the covalent attachment of substrate proteins to the cell walls of gram-positive bacteria, and thereby play a crucial role in virulence, infection and colonisation by pathogens. Many cell-surface proteins are anchored by the housekeeping sortase SrtA but other more specialised sortases exist that attach sub-sets of proteins or function in pilus assembly. The sortase Spy0129, or SrtC1, from the M1 SF370 strain of Streptococcus pyogenes is responsible for generating the covalent linkages between the pilin subunits in the pili of this organism. The crystal structure of Spy0129 has been determined at 2.3 Å resolution (R = 20.4%, Rfree = 26.0%). The structure shows that Spy0129 is a class B sortase, in contrast to other characterised pilin polymerases, which belong to class C. Spy0129 lacks a flap believed to function in substrate recognition in class C enzymes and instead has an elaborated β6/β7 loop. The two independent Spy0129 molecules in the crystal show differences in the positions and orientations of the catalytic Cys and His residues, Cys221 and His126, correlated with movements of the β7/β8 and β4/β5 loops that respectively follow these residues. Bound zinc ions stabilise these alternative conformations in the crystal. This conformational variability is likely to be important for function although there is no evidence that zinc is involved in vivo.
- Maurice Wilkins Centre for Molecular Biodiscovery, University of Auckland, Auckland, New Zealand.
Organizational Affiliation: