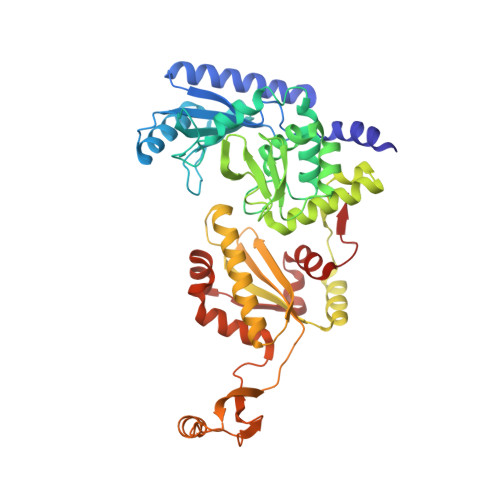
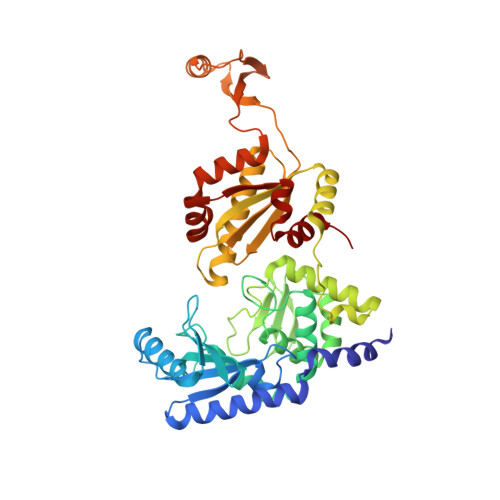
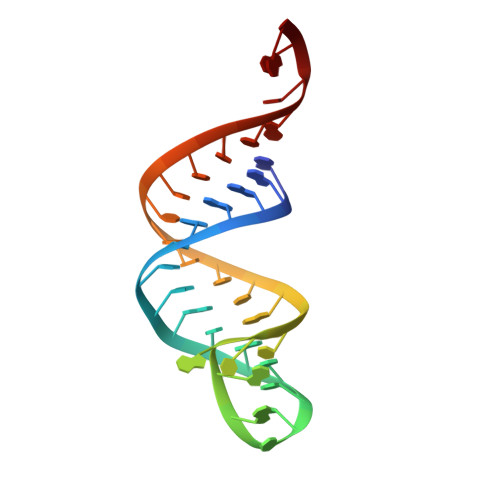
How the CCA-Adding Enzyme Selects Adenine over Cytosine at Position 76 of tRNA.
Pan, B., Xiong, Y., Steitz, T.A.(2010) Science 330: 937-940
- PubMed: 21071662
- DOI: https://doi.org/10.1126/science.1194985
- Primary Citation of Related Structures:
3OUY, 3OV7, 3OVA, 3OVB, 3OVS - PubMed Abstract:
CCA-adding enzymes [ATP(CTP):tRNA nucleotidyltransferases] add CCA onto the 3' end of transfer RNA (tRNA) precursors without using a nucleic acid template. Although the mechanism by which cytosine (C) is selected at position 75 of tRNA has been established, the mechanism by which adenine (A) is selected at position 76 remains elusive. Here, we report five cocrystal structures of the enzyme complexed with both a tRNA mimic and nucleoside triphosphates under catalytically active conditions. These structures suggest that adenosine 5'-monophosphate is incorporated onto the A76 position of the tRNA via a carboxylate-assisted, one-metal-ion mechanism with aspartate 110 functioning as a general base. The discrimination against incorporation of cytidine 5'-triphosphate (CTP) at position 76 arises from improper placement of the α phosphate of the incoming CTP, which results from the interaction of C with arginine 224 and prevents the nucleophilic attack by the 3' hydroxyl group of cytidine75.
- Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA.
Organizational Affiliation: