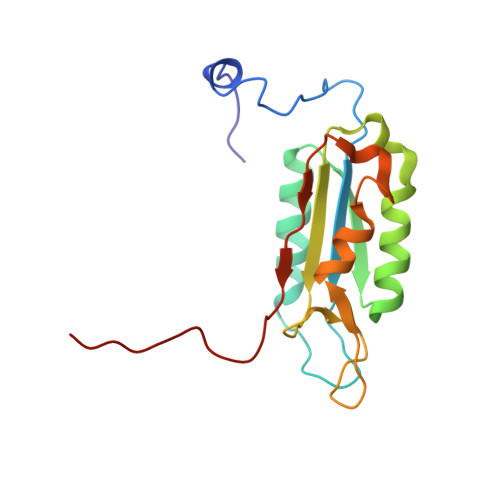
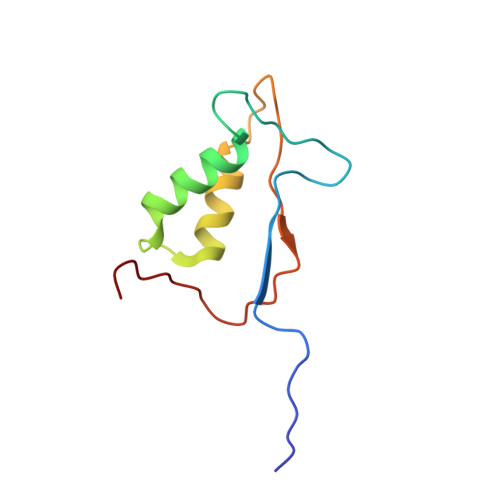
Succinic acid amides as P2-P3 replacements for inhibitors of interleukin-1beta converting enzyme (ICE or caspase 1).
Galatsis, P., Caprathe, B., Gilmore, J., Thomas, A., Linn, K., Sheehan, S., Harter, W., Kostlan, C., Lunney, E., Stankovic, C., Rubin, J., Brady, K., Allen, H., Talanian, R.(2010) Bioorg Med Chem Lett 20: 5184-5190
- PubMed: 20656488
- DOI: https://doi.org/10.1016/j.bmcl.2010.07.004
- Primary Citation of Related Structures:
3NS7 - PubMed Abstract:
Succinic acid amides have been found to be effective P2-P3 scaffold replacements for peptidic ICE inhibitors. Heteroarylalkyl fragments occupying the P4 position provided access to compounds with nM affinities. Utilization of an acylal prodrug moiety was required to overcome biopharmaceutical issues which led to the identification of 17f, a potential clinical candidate.
- Pfizer Global R&D, Ann Arbor, MI 48105, USA. paul.galatsis@pfizer.com
Organizational Affiliation: