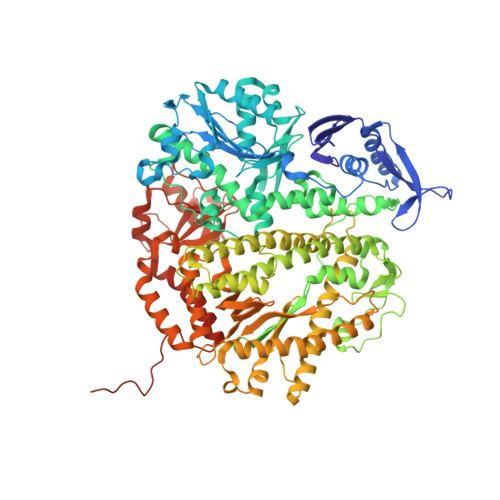
Variation in Mutation Rates Caused by RB69pol Fidelity Mutants Can Be Rationalized on the Basis of Their Kinetic Behavior and Crystal Structures.
Xia, S., Wang, M., Lee, H.R., Sinha, A., Blaha, G., Christian, T., Wang, J., Konigsberg, W.(2011) J Mol Biology 406: 558-570
- PubMed: 21216248
- DOI: https://doi.org/10.1016/j.jmb.2010.12.033
- Primary Citation of Related Structures:
3NDK, 3NE6, 3NGI, 3NHG - PubMed Abstract:
We have previously observed that stepwise replacement of amino acid residues in the nascent base-pair binding pocket of RB69 DNA polymerase (RB69pol) with Ala or Gly expanded the space in this pocket, resulting in a progressive increase in misincorporation. However, in vivo results with similar RB69pol nascent base-pair binding pocket mutants showed that mutation rates, as determined by the T4 phage rI forward assay and rII reversion assay, were significantly lower for the RB69pol S565G/Y567A double mutant than for the Y567A single mutant, the opposite of what we would have predicted. To investigate the reasons for this unexpected result, we have determined the pre-steady-state kinetic parameters and crystal structures of relevant ternary complexes. We found that the S565G/Y567A mutant generally had greater base selectivity than the Y567A mutant and that the kinetic parameters for dNMP insertion, excision of the 3'-terminal nucleotide residue, and primer extension beyond a mispair differed not only between these two mutants but also between the two highly mutable sequences in the T4 rI complementary strand. Comparison of the crystal structures of these two mutants with correct and incorrect incoming dNTPs provides insight into the unexpected increase in the fidelity of the S565G/Y567A double mutant. Taken together, the kinetic and structural results provide a basis for integrating and interpreting in vivo and in vitro observations.
- Department of Molecular Biophysics and Biochemistry, Yale University, 333 Cedar Street, New Haven, CT 06520, USA.
Organizational Affiliation: