Crystal Structure of PsbQ from Synechocystis sp. PCC 6803 at 1.8 A: Implications for Binding and Function in Cyanobacterial Photosystem II
Jackson, S.A., Fagerlund, R.D., Wilbanks, S.M., Eaton-Rye, J.J.(2010) Biochemistry 49: 2765-2767
- PubMed: 20210304
- DOI: https://doi.org/10.1021/bi100217h
- Primary Citation of Related Structures:
3LS0, 3LS1 - PubMed Abstract:
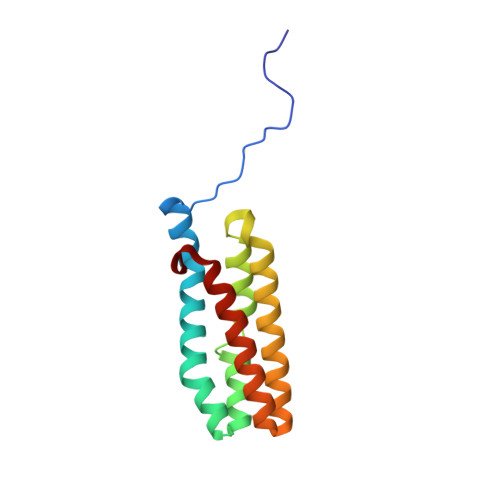
In Synechocystis sp. PCC 6803, PsbQ is associated with photosystem II (PSII) complexes with the highest activity and stability. However, this subunit is not found in PSII X-ray crystallographic structures from Thermosynechococcus elongatus or Thermosynechococcus vulcanus. We present the crystal structure of cyanobacterial PsbQ determined in the presence and absence of Zn(2+). The protein has a well-defined helical core, containing four helices arranged in an up-down-up-down fold. A conserved potential interaction site composed of a divalent metal binding site and adjacent hydrophobic pocket has been identified.
- Department of Biochemistry, University of Otago, Dunedin 9054, New Zealand.
Organizational Affiliation: